Mastering the fundamentals of Alkyd Resin Technology
Although alkyds are no longer the largest volume resin type used in coatings, they still play a significant role in the coatings industry, not only because of their versatility, but also because they employ a significant amount of renewable material.
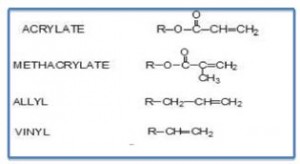
The term alkyd is derived from alcohol and acid.
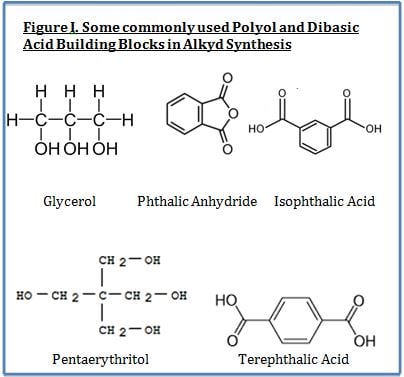
Alkyds are prepared from the condensation reaction between polyols, dibasic acids and fatty acids. The fatty acid portion is derived from vegetable matter and thus is a renewable resource. Key performance features of alkyds include their ability to offer improved surface wetting (from the bio-based fatty acid portion of substrates and pigments) and lower cost (also primarily from the fatty acid portion). The most widely used polyols include glycerol, pentaerythritol and trimethyol propane whereas the most widely used dibasic acids are phthalic anhydride and isophthalic acid.
Naturally-occurring oils are in the form of triglcerides. Triglycerides are triesters of glycerol and fatty acids. Triglycerides can be drying oils, but many are not. The reactivity of drying oils with oxygen results in 1,4 –dienes. The naturally-occurring oils are comprised of mixtures of mixed triglycerides with different fatty acids as part of the glyceride molecules.
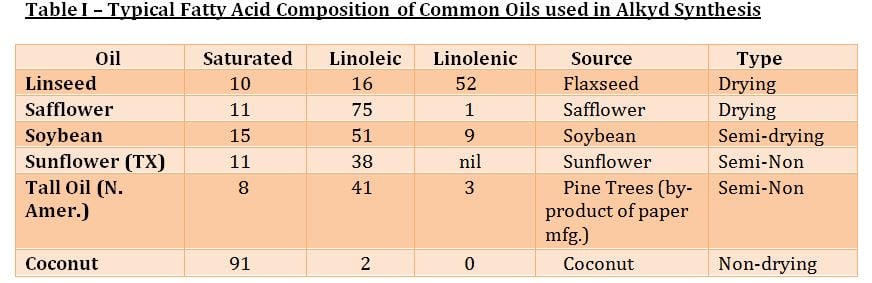
Some of these glyceride molecules are comprised of a higher percentage of fatty acids with a greater amount of non-conjugated unsaturation with diallylic methylene groups and result in improved drying capability. For example, linoleic acid has one active diallylic group (-CH=CH – CH2 – CH=CH -), whereas linolenic has two active methylene groups. Also, to increase drying speed, alkyds can be modified with vinyl toluene or styrene to increase the Tg and thus reduce the time required to reach a given hardness. If the amount of oil in an alkyd is over 60%, it is called a long oil alkyd. If it’s between 40 and 60%, it’s known as a medium oil alkyd, and those with less than 40 are considered short oil alkyds. The formula for calculating the percent oil length based on the amount of fatty acid is as follows:
In addition to the amount of oil as well as the selection of the alcohol and acid functional components, the type of oil has a profound effect on the dry time and performance.
Fatty acids are further categorized into drying, semidrying and non-drying. Non-conjugated oils are considered drying oils if their drying index, as calculated as follows, is more than 70. The higher the amount ofLinolenic and Linoleic content, the higher the drying index:
Although drying speed is improved as the % linolenic increases, the rate of yellowing for exterior white coatings is also greater. Accordingly, alkyds using safflower and sunflower oils which provide improved resistance to yellowing as a result of their lower linolenic content.
In addition to classifying alkyds by their oil length and the type of fatty acid present, alkyds are also classified into oxidizing and non-oxidizing categories. Oxidizing alkyds crosslink through a complex multistage auto-oxidation mechanism, whereas Non-oxidizing alkyds do not crosslink and are thus thermoplastic unless their available hydroxyl groups are crosslinked with an aminoplast (heat cured) or isocyanate crosslinker (ambient cured).
To read the rest of the article, written by Chemical Dynamics’ President, Ron Lewarchik, click over to UL Prospector here.