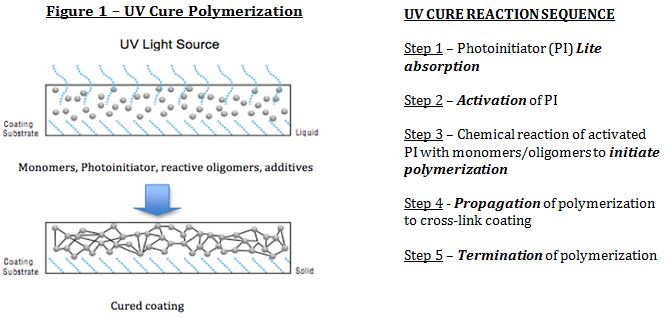
UV-LED Curable Coatings offer a high-speed light curing process with a number of advantages over more conventional cure processes. Multiple advantages include High speed, lower energy requirements, little or no VOC, less production space, less dirt collection, high quality finish, rapid processing as well as instant on-off with some UV light technologies also expedite production and energy savings. UV Curable paint finishes have existed since the 1960’s and are based on polymerization reactions including free radical and cation-initiated chain-growth polymerization. As the majority of coatings for UV cure coating utilize free radical polymerization (>90% of market), this article will focus primarily on free radical polymerization initiated by a photoinitiator (Fig. 1):
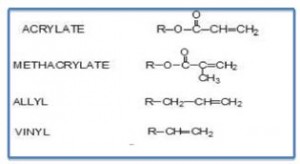
The types of unsaturation used in UV/EB cure coatings are provided in Table I, with by far the largest type being acrylate.
Photoinitiator
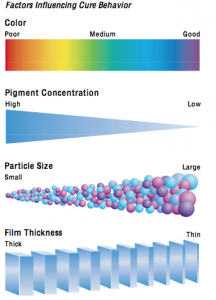
considerations primarily include two different characteristics of the photoinitiator’s absorption curve. First, is the maximum wavelength (Lambda Max) of light that is absorbed by the PI and second, the strength of this absorption (molar extinction coefficient). Photoinitiators developed for curing pigmented films normally have higher molar extinction coefficients at longer wavelengths between 300 nm to 450 nm than those for curing clear formulations. To maximize cure and efficiency, the PI’s absorbance must match the light output of the lamp as different lamps have different spectral outputs (see Table I). Longer wave- length light is also essential to enhance cure in thicker coatings. Newer PI’s have also enabled the formulation of pigmented coatings in addition to that of clear coatings. The general cure considerations influenced by color, PVC, pigment particle size and film thickness are summarized in Fig. 2:
There are two main types of free radical photoinitiators, Type I and Type II. Type I photoinitiators undergo cleavage upon irradiation to form two free radicals. Normally only one of these free radicals is reactive and thus initiates polymerization. 1-hydroxy-cyclohexylphenyl-ketone is a widely used Type I PI. Type II photoinitiators form an excited state upon irradiation, and abstract an atom or electron from a donor molecule (synergist). The donor molecule in turn initiates polymerization. An example of a widely used Type II photoinitiator is benzophenone. Tertiaryamines are typically used as synergists as they react with benzophenone, and also retard the inhibition of polymerization by oxygen. Acrylated tertiary amine compounds are used when odor and extractables are of concern. Oxygen can also inhibit cure especially in thin films; to counteract oxygen inhibition, coatings can use amine synergists, be cured under a nitrogen atmosphere, employ the addition of wax, high initiator concentration, more intense UV Light, and/or surface active initiators.
To read the rest of the article, written by Chemical Dynamics’ President, Ron Lewarchik, click over to UL Prospector here.