The global electrocoat market is estimated to grow from USD 3.08 billion in 2016 to USD 3.80 billion by 2021, at a CAGR of 4.34 percent between 2016 and 2021. This market is witnessing moderate growth because of demand for e-coat from the end-use industries such as automotive and appliances1.
Electrocoating is a process that uses an electrical current to deposit an organic coating from a paint bath onto a part or assembled product. Due to its ability to penetrate recesses and thus coat complex parts and assembled products with specific performance requirements, electrocoat is used worldwide throughout various industries to coat a wide swath of products including those in automotive, appliance, marine and agriculture. Electrocoat, and specifically cathodic electrocoat, has enabled a dramatic improvement in corrosion resistance over that offered by anodic electrocoat or other more conventional methods of coating.
Cathodic electrocoat is available in multiple technology types, most notably is epoxy cathodic and acrylic cathodic.
- Acrylic cathodic is expected to experience the highest growth in the e-coat market. Cathodic acrylic e-coat is typically used in applications that require UV durability as well as corrosion protection on ferrous substrates. It is also used in applications where light colors are required.
- Cathodic acrylic e-coat is available in a wide range of glosses and colors to provide both exterior weathering and corrosion protection. Acrylic cathodic is used as a one-coat finish for agricultural implements, garden equipment, appliances and exterior HVAC.
The application of electrocoat involves four steps:
- Pretreatment2: After the parts are cleaned, a pretreatment is applied to prepare the metal surface for electrocoating.
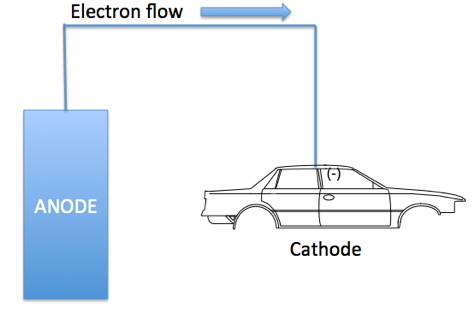
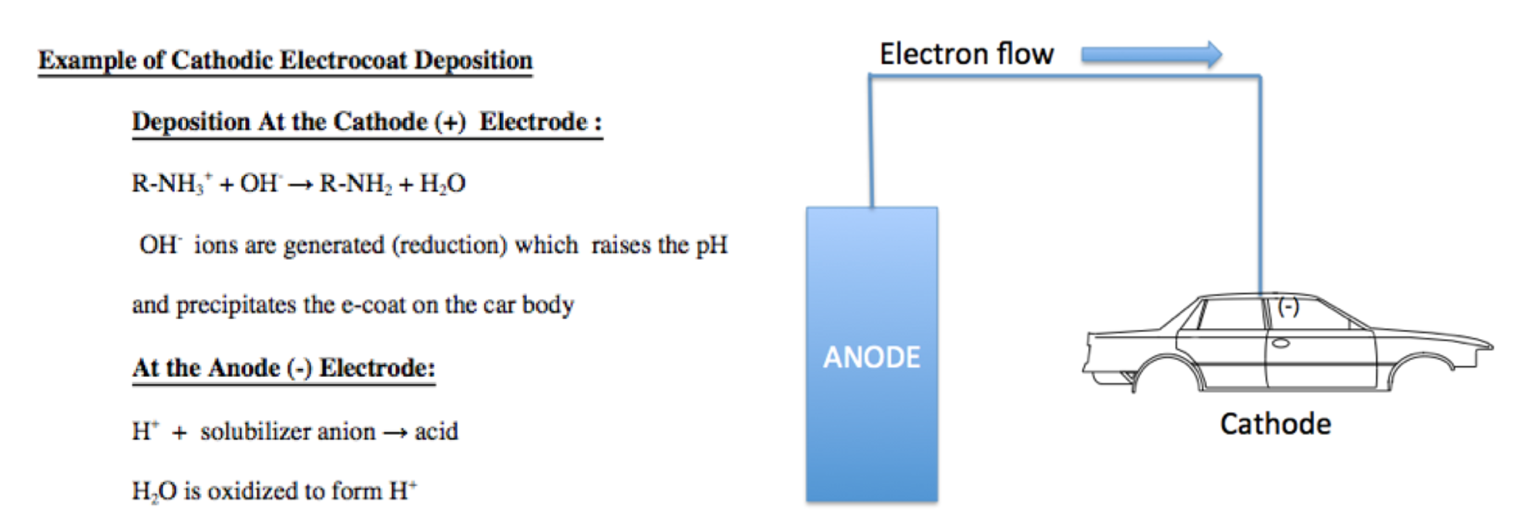
- Electrocoat Application: Positively charged cathodic paint is deposited on the electrically conductive substrate from a cathodic paint bath using direct current. The positively charged paint is deposited at the negatively charged cathode where reduction takes place.
- Post Rinses: Parts are rinsed to reclaim undeposited paint solids.
- Bake: Paint is baked to thermally cross-link the paint and volatilize water as well as any residual organic solvents.
In step 2, paint particles are deposited on the surface of the electrically conductive substrate to form an insulating film. The rate of the film deposited diminishes with time as the conductivity of the paint surface has an insulating effect as the film increases in film thickness. At this point the deposited film has very little water and solvent present so the water post rinses (step 3) do not have a negative effect on the deposited film. The coated substrate is then baked to eliminate water and remaining volatile as well as to crosslink the polymeric film.
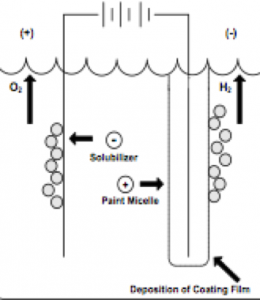
As figure I indicates, in cathodic electrodeposition, the positively charged paint is attracted to the negatively charged cathode where reduction occurs, resulting in the liberation of hydrogen gas. At the anode, oxidation occurs with the accompanying release of oxygen. The deposited paint film is coalesced into a relatively insoluble paint film and after one or more water rinses, the deposited paint film enters a bake oven to enable the crosslinking of the cathodic paint film.
The advantages and disadvantages of cathodic electrocoat include:
- Excellent corrosion resistance, even at lower film thicknesses
- Offers excellent resistance to bimetallic corrosion (when dissimilar metals are in contact)
- Frequent color change is not practical
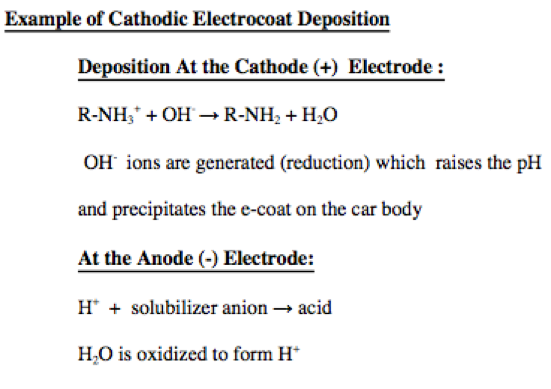
Many cationic epoxy electrocoat resins are comprised of a Bisphenol A based epoxy resin comprised of amine groups that are neutralized with a low molecular weight acid such as formic, acetic or lactic acid. Since the coating bath has a pH of slightly below 7, bath components are comprised of stainless steel or other corrosion resistant materials to prevent rust formation.
The most common crosslinker is a blocked isocyanate, so once the coating is baked, the blocked isocyanate is activated and reacts with available hydroxyl and amine groups. Other components of a typical electrocoat bath include pigment, filler pigment, water, solvent and a low level of modifying resins such as plasticizers and flexibilizers, flow modifiers and catalysts.
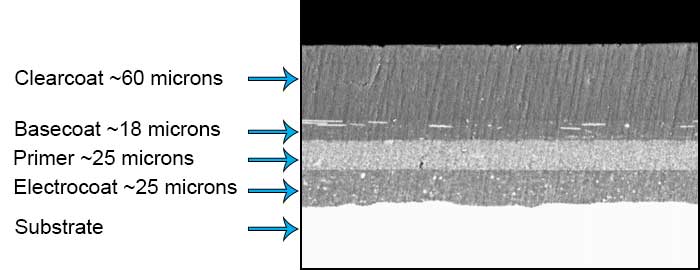
Ecoat is used because it provides superior corrosion protection as it coats surfaces that are inaccessible by conventional means. Film thickness is uniform without any defects such as sags, runs or edge beads. Electrocoat is also very cost effective as it provides nearly 100 percent material utilization with good energy efficiency and a relatively low cost per square foot of applied coating.
Throwpower is the ability of an electrocoat to penetrate into “hard to reach” areas, such as the inside of a hollow metal object. Dependent on applied voltage, bath solids, conductivity, deposition time, bath temperature, solvent levels, and proper tank agitation, deposition time, throwpower and coating appearance can be optimized.
A simple dip-applied coating cannot effectively coat the interior of complex shaped parts, as during the bake process, the water/solvent has a washing effect in the interior portions of the part that prevents adequate film build. At the time an electrocoated object is removed from the bath, most of the water and solvent is squeezed from the electrocoat so that during the bake the washing effect is minimal as compared to that of a simple dip-applied coating.
The film build of electrocoat paint is self-limiting as the film becomes more insulative as the thickness of the film approaches its maximum. Higher voltage and longer immersion times will permit higher film builds until the maximum possible film build is reached, which is normally about 1.0 mil and 1.2 mils.
Voltage is normally between 225 and 400 volts. If the voltage is too high, there will be film rupture of the coating applied to the outer surfaces. This is called the rupture voltage. At a sufficiently high voltage, the current will break through the film, leading to gas generation under the film (hydrogen for cathodic and oxygen for anodic).
Other factors that affect film build include bath temperature and conductivity. Immersion times are normally on the order of 2-3 minutes.
In summary cationic electrocoating is expected to grow at a faster rate than that of more conventional product finishing processes as it provides excellent corrosion protection for complex shapes, low volatile organic content and lastly acrylic cationic electrocoat also offers resistance to UV light for experior applications.
To read the rest of the article please click here to head over to UL Prospector.
__________
Ron Lewarchik, Author of article & President of Chemical Dynamics
As a contributing writer, Ron pens articles on topics relevant to formulators in the coatings industry. He also serves as a consultant for the Prospector materials search engine, advising on issues related to optimization and organization materials within the database.





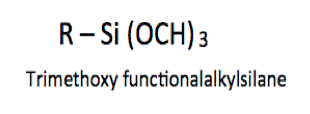 The trialkoxy silanes that are discussed in this article are those that contain primarily
The trialkoxy silanes that are discussed in this article are those that contain primarily