Radiation cure coatings offer a high-speed light curing process with a number of advantages over more conventional cure processes. Multiple advantages include high speed, lower energy requirements, little- or no-VOC, less production space, less dirt collection, high quality finish, rapid processing as well as instant on-off with some UV light technologies also expedite production and energy savings. Electron and UV curable paint finishes have existed since the 1960s and are based on polymerization reactions including free radical and cation-initiated chain-growth polymerization. As the majority of coatings for UV cure coating utilize free radical polymerization (>90% of market), this article will focus primarily on free radical polymerization initiated by a photoinitiator (Fig. 1):
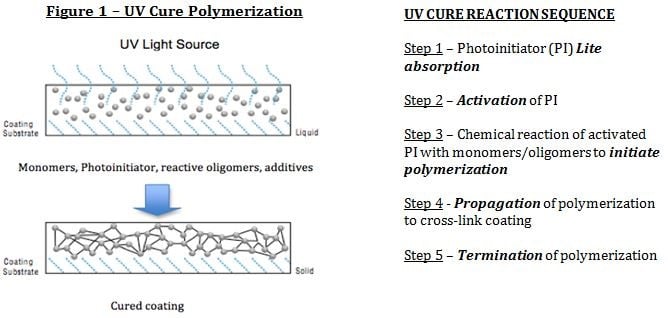
The types of unsaturation used in UV/EB cure coatings are provided in Table I, with by far the largest type being acrylate.
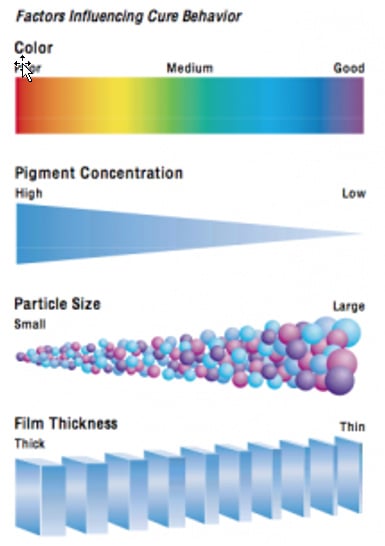
Photoinitiator considerations primarily include two different characteristics of the photoinitiator’s absorption curve. First, is the maximum wavelength (Lambda Max) of light that is absorbed by the PI and second, the strength of this absorption (molar extinction coefficient). Photoinitiators developed for curing pigmented films normally have higher molar extinction coefficients at longer wavelengths between 300 nm to 450 nm than those for curing clear formulations. To maximize cure and efficiency, the PI’s absorbance must match the light output of the lamp as different lamps have different spectral outputs (see Table I). Longer wavelength light is also essential to enhance cure in thicker coatings. Newer PI’s have also enabled the formulation of pigmented coatings in addition to that of clear coatings. The general cure considerations influenced by color, PVC, pigment particle size and film thickness are summarized in Fig. 2:
There are two main types of free radical photoinitiators, Type I and Type II. Type I photoinitiators undergo cleavage upon irradiation to form two free radicals. Normally only one of these free radicals is reactive and thus initiates polymerization. 1-hydroxy-cyclohexylphenyl-ketone is a widely used Type I PI. Type II photoinitiators form an excited state upon irradiation and abstract an atom or electron from a donor molecule (synergist). The donor molecule in turn initiates polymerization. An example of a widely used Type II photoinitiator is benzophenone. Tertiary amines are typically used as synergists as they react with benzophenone, and also retard the inhibition of polymerization by oxygen. Acrylated tertiary amine compounds are used when odor and extractables are of concern. Oxygen can also inhibit cure especially in thin films; to counteract oxygen inhibition, coatings can use amine synergists, be cured under a nitrogen atmosphere, employ the addition of wax, high initiator concentration, more intense UV Light, and/or surface-active initiators.
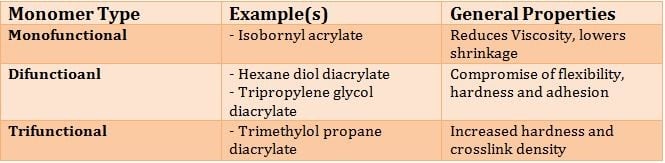
Other key ingredients that determine the performance of UV Cure formulations include UV curable monomers and oligomers. Figure 3 illustrates typical monomers that are used along with performance characteristics.
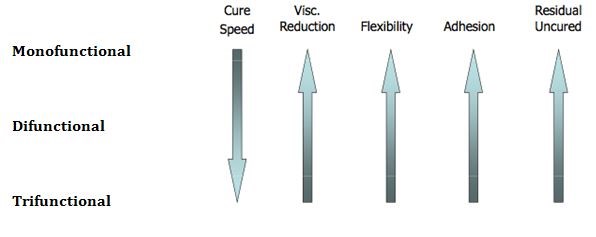
Table II. General Performance Versus Monomer
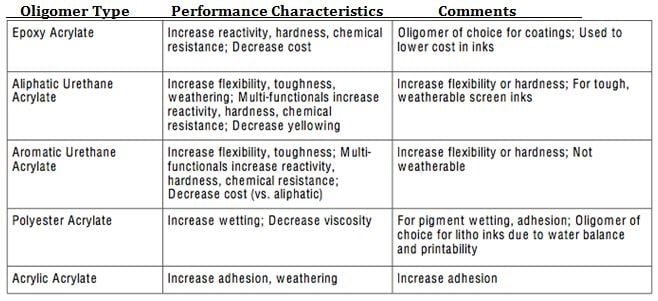
There are a number of UV curable oligomer types available as well depending on the type of performance desired, please refer to Fig. 4 for a listing of some of the common oligomer types available along with an overview of performance characteristics.
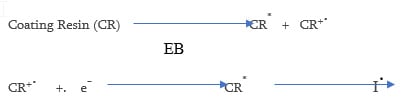
Electron Beam cure coatings can be used to cure acrylate functional coatings. As the energy used is much higher (150 – 300 keV) than that in UV cure, a photoinitiator is not necessary. Other advantages of EB cure over UV is that pigments do not adversely effect the cure. The vehicle systems that are used are essentially the same as that used in UV curing (acrylates) and vehicles used for UV cationic cure. Disadvantages of EB curing include the high cost of capital equipment and curing must be done in an inert environment. The coating resin once irradiated forms a radical cation and a secondary electron the excited state CR* can then homolytically cleave to form a free radical and initiate polymerization.
In addition to 100% solid liquid UV coatings, other UV types include waterborne UV and powder UV. Waterborne UV curables have advantages over conventional UV cure as no reactive diluent is necessary to control viscosity. Also, as opposed to conventional UV cure formulations, the viscosity of the coating is independent of the molecular weight of the resin and for spray application viscosity, solids are adjusted by adding water rather than low viscosity reactive monomer. In addition, since there are fewer double bonds to cure, shrinkage is lower and can thus improve adhesion. The main disadvantage is that the water needs to be removed by passing through an oven at about 80°C prior to UV curing. In powder UV cure coatings, the part is sprayed electrostatically. Automatic guns are recommended over manual application to ensure an even, consistent film thickness is applied. Next, the applied coating is baked in a convection, IR or oven to melt and flow the powder. This step is at a much lower temperature and less time (175-280 °F for a few seconds instead of 320-390°F for 5 to 20 minutes) for conventional powder coating. Once the powder is melt flowed, the parts enter a UV cure chamber that cure the coating in seconds instead of minutes, as with traditional thermal powder.
Cationic UV cure coatings have the following advantages:
- Low shrinkage
- Excellent adhesion
- Not inhibited by oxygen
- Dark cure permits a high level of conversion
- Improved physical properties
Typical photoinitiators for cationic cure coatings are typically onium salts of strong acids such as iodonium and sulfonium salts of hexafluoroantimonic and hexafluorophosphoric acids. Once exposed to suitable irradiance in the range of 200 – 360nm a strong Bronsted acid is activated which acts a catalyst for the homopolymerization of oxirane functional groups on the reactants. Normally cycloaliphatic epoxies are used as they react faster that aromatic based epoxies such as

Tetrakis pentafluorophenyl borate anion is fastest in the above group (lowest nucleophilicity). The addition of photosensitizers such as thioxanthones, benzophenone and anthracenes can enhance a spectral response into the mid-visible energy range to improve reaction efficiency. From a vehicle standpoint, cationic UV cure coatings use cycloaliphatic epoxies as they are faster reacting than those utilizing aromatic epoxies based on BPA. Onium salts can also be used as phtoinitiators for hybrid free radical-cationic polymerization. Hybrid radical-cationic coatings use cycloaliphatic epoxies in addition to reactants such as vinyl ethers, styrene, and 4-alkoxystyrene. As moisture acts as chain transfer agent in cationic cure, cure speeds decrease dramatically above 50% R.H. After activation of the cationic photoinitiator, the polymerization reactions are thermally driven. This accounts for a high conversion rate especially in the presence of a thermal bump.
Any discussion of UV-LED cure coatings is remiss without at least a short overview of UV-LED bulbs as well as the characteristics of each type. As illustrated in Table III.
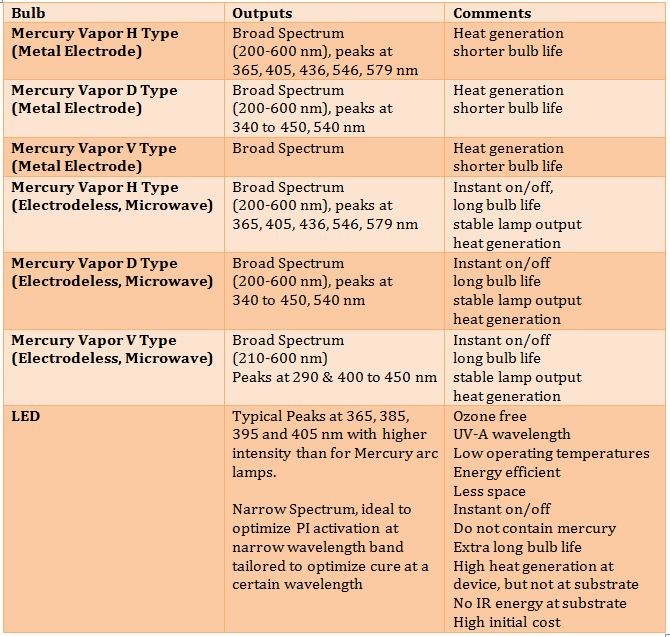
A final consideration of UV cure coatings is that they are normally line of sight. In other words, for complex three-dimensional surfaces, where the light does not shine, the coating will not cure. Also, most UV cure technologies provide optimum uniform cure on a two-dimensional surface using focused light. LED curing has multiple advantages over more traditional UV cure technology such as low heat generation. This is ideal for curing heat sensitive substrates. In addition, LED offers an ozone free environment, energy efficiency, and ultralong bulb life and the stable spectral output means consistent quality.
