Most polyester resins used in coating applications are relatively low molecular weight and are amorphous, linear or branched and must be crosslinked to form useful films. As a class, thermosetting polyesters generally provide better metal adhesion and impact resistance than thermosetting acrylics, however TSA’s provide coatings with better resistance to hydrolysis and weathering. The presence of ester linkages in the backbone of polyesters make them more prone to hydrolysis, proper selection of backbone monomers that provide steric hindrance to the ester group linkage (for example NPG provides improved resistance to hydrolysis and weather resistance.
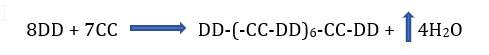
This article will only consider saturated polyesters which are sometimes referred to as oil free polyesters. Polyester coatings are a large portion of the construction, automotive and aerospace markets as they can be engineered to provide excellent properties including mechanical, impact, UV, and chemical resistance for use in waterborne, high solids low VOC and powder coatings. Linear polyesters account for a large portion of the resins used for coil coating applications. When cured with melamine or blocked isocyanate can provide excellent flexibility, chemical resistance and light stability. Formation of polyesters is accomplished by step-growth polymerization of an alcohol with at least two hydroxy groups and a carboxylic acid with at least two carboxyl groups. Most often polyesters contain a blend of diols, triols and dibasic acid with an excess of polyol to form a hydroxy terminated polyester for reaction with melamine or isocyanate prepolymer to form a coating film. If an excess of dibasic acid is used, the polyester is carboxy terminated for reaction with epoxy, melamine or 2-hydroxyalkylamides. Historically polyester synthesis was referred to as condensation polymerization as the reaction of an alcohol group and a carboxyl group produces water. Other polyester synthesis routes include the reaction of an ester with an alcohol, the reaction of an anhydride and an alcohol and lastly the ring opening polymerization of a lactone. When a diol (DD) reacts with a dibasic acid (CC) in equal molar amounts, the molecular weight builds gradually and is more readily controlled. The reactant in excess will have terminal groups of that reactant. For example:
The average molecule will have terminal hydroxyl groups. Branched polyesters are made from mixtures of monomer that contain one or more monomers which have a functionality F > 2. As the proportion of a monomer with F (functionality) > 2 increases, the Number Average Molecular weight increases and the reaction must be controlled to avoid gelation. A wide range of polyesters are in commercial use, for conventional polyesters cured with melamine or isocyanate prepolymers, the number average molecular weight is in the 2,000 to 6,000 range.
Figure 1 – Increase in molecular weight during polyester synthesis:
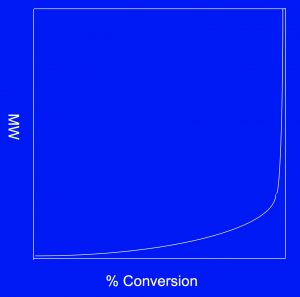
Figure 2 – Common hydroxyl functional monomers are as follow:
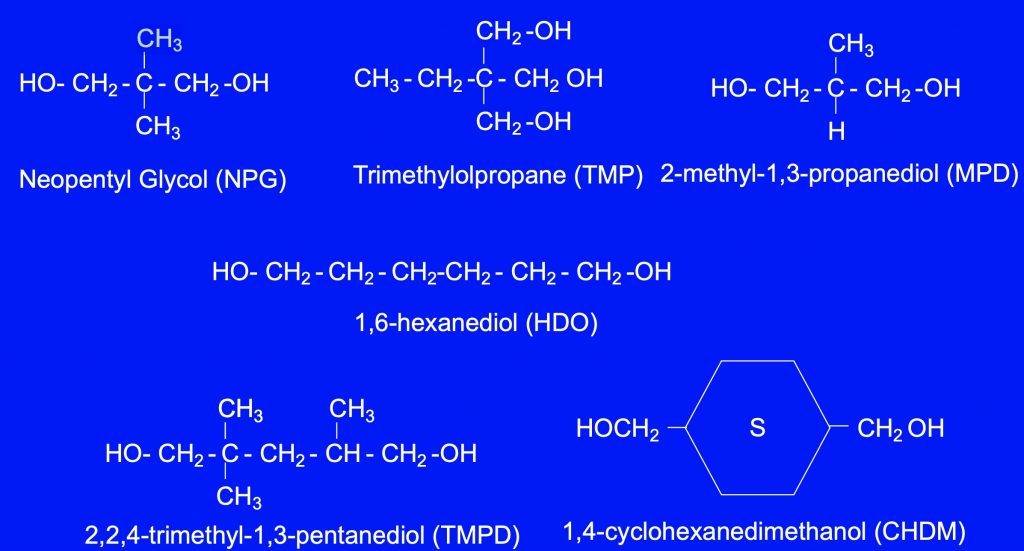
Figure 3 – Common Diacid monomers:
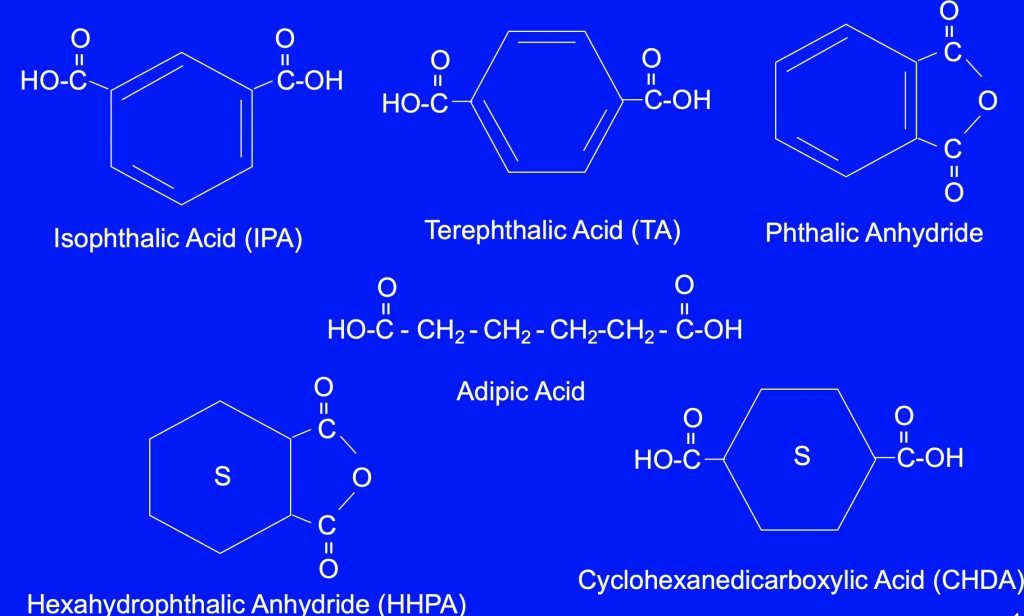
Table I – Effect of polyols on Polymer Properties:
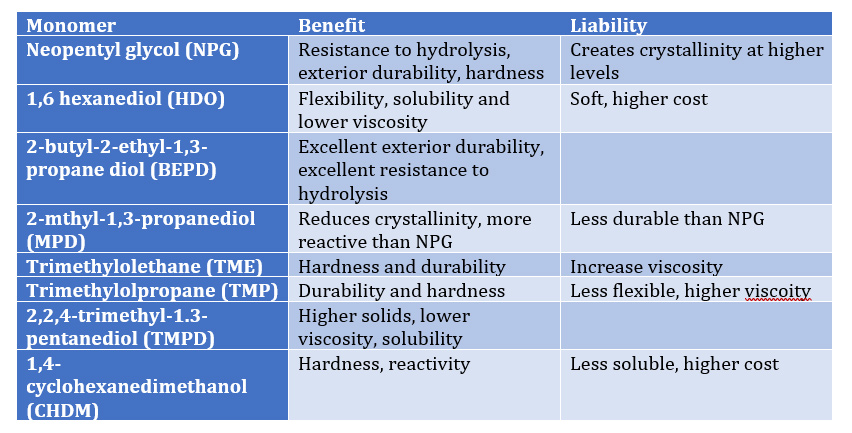
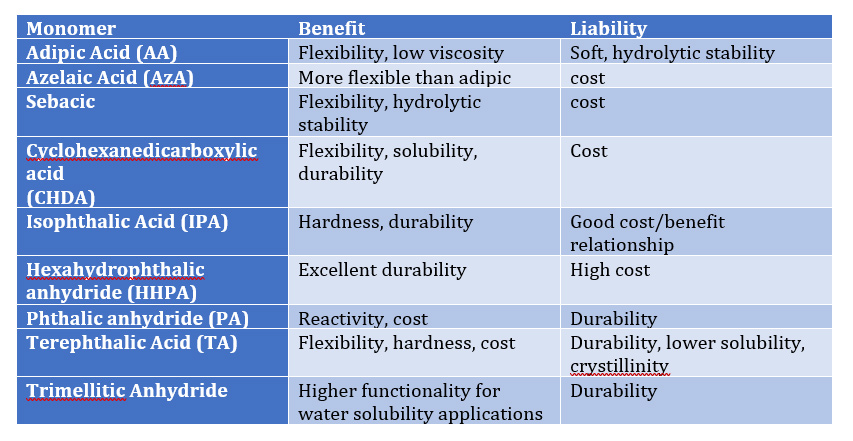
Table II – Effect of acid functional monomers on Polymer Properties:
As Tables I and II illustrate, proper selection of co reactant monomers can provide a range of performance characteristics to provide an array of performance attributes such as
- hydrolytic stability (NPG, Sebacic, CHDA)
- exterior weathering (NPG, BEPD, TMP, TME, HHPA, IPA)
- hardness ( NPG, TME, TME, CHDM, TA)
- flexibility (AA, AzA, Seb, CHDA, TA, CDO)
Desired performance can be achieved through the proper selection of a blend of monomers coupled with the selection of the polymer architecture to meet film performance properties.
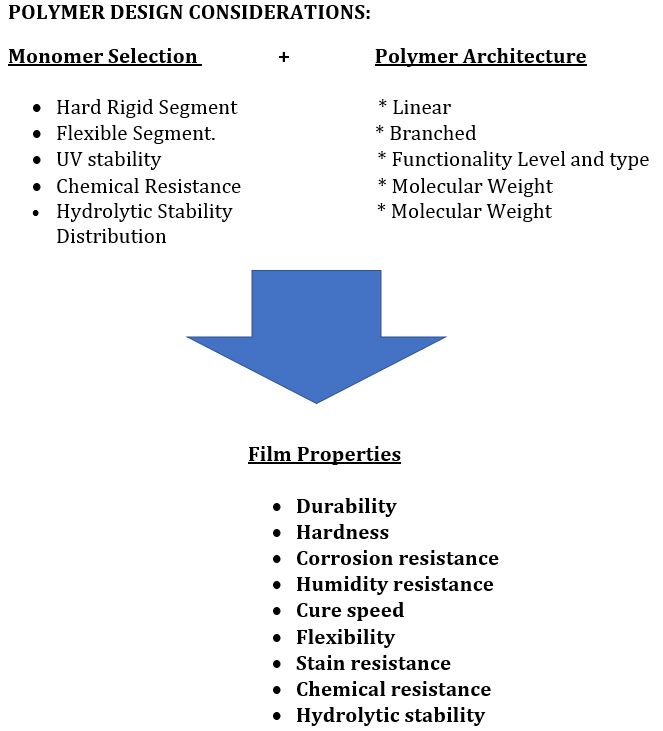
Lastly, the architecture of polyesters can be modified with one or more reactive moieties to form for example urethane, oil, or acrylic modified polyesters.
For additional information concerning polyesters, bio-based resins and raw materials, please navigate to www.ulprospector.com.
Resources:
- Organic Coatings, Science and Technology, Frank N. Jones et.al., Wiley & Sons, 2017
- Prospector
