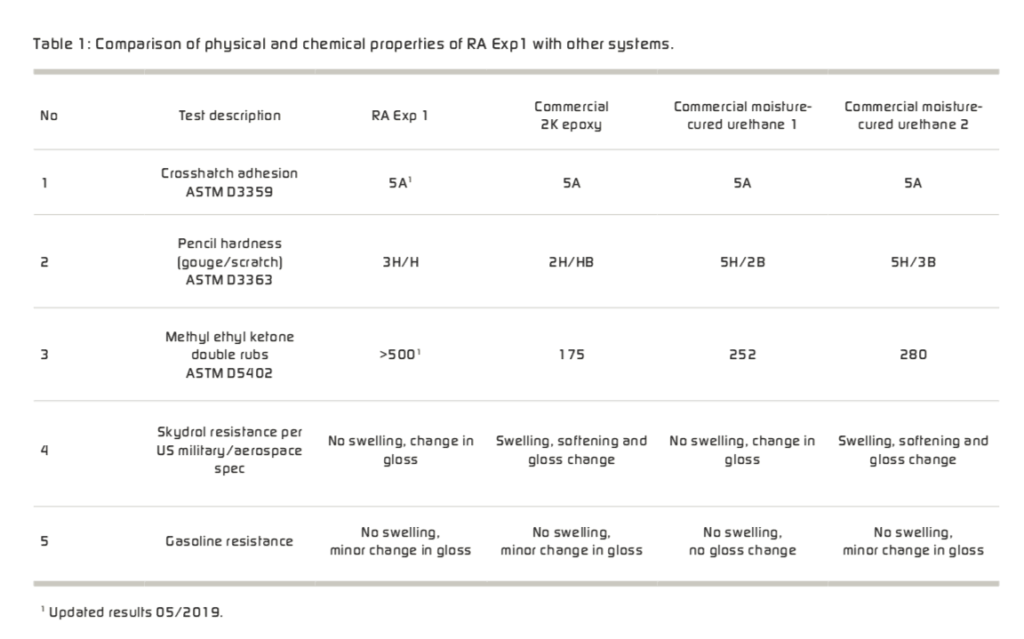
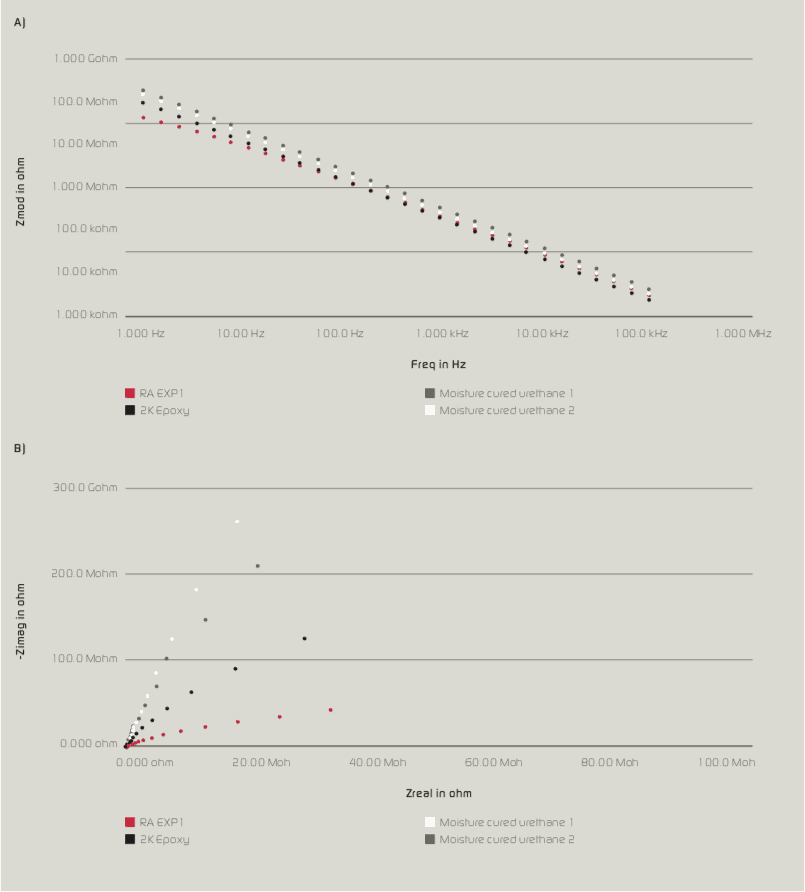
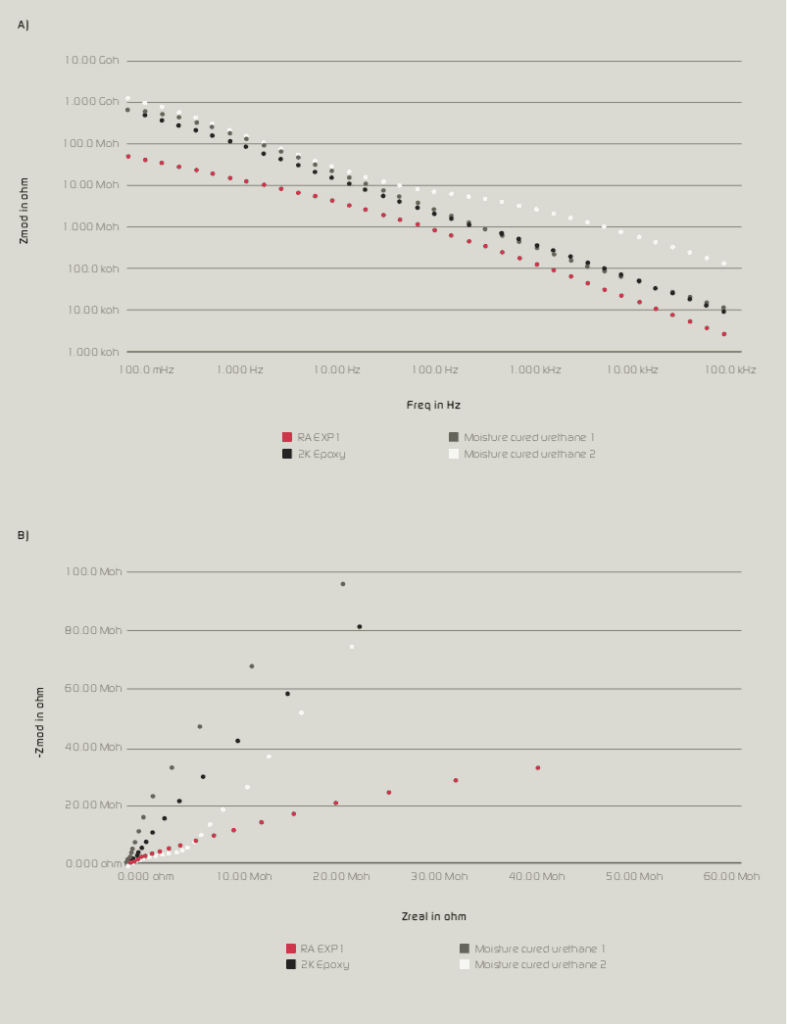
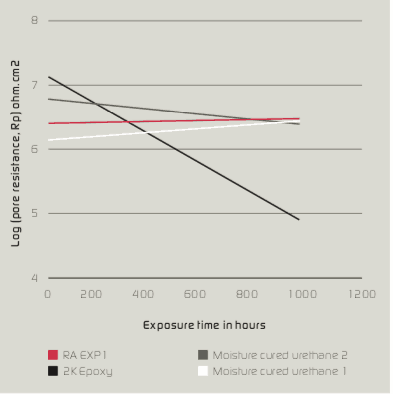
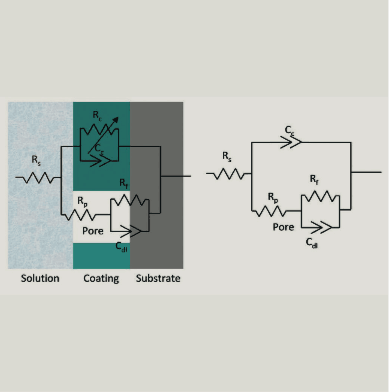
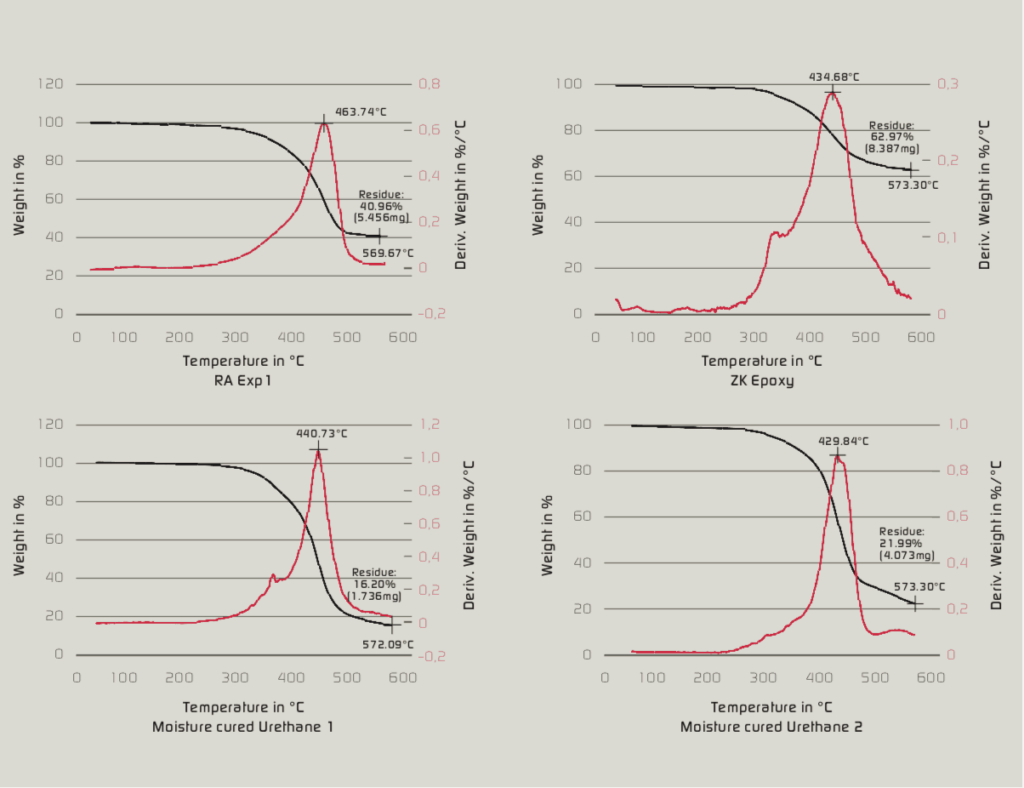
Direct to Metal Coatings (DTM) is a rapidly growing segment of the coatings industry. This growth is related to cost reduction attributed to improved efficiency, time savings and fewer production steps. These coatings are used in the heavy construction industry, building products and product finishing. Many of these applications require performance in demanding exposure conditions such as oil drilling, off shore oil rigs and foundries. The compound annual growth rate of DTM coatings is estimated to be about 10%. DTM coatings are applied by spray, brush, roll and coil coating. Substrates include aluminum, cold rolled steel, hot rolled steel and coated metals (e.g. hot dip galvanized steel, galfan, galvalume, electrogalvanized steel and plated metals).
By definition, DTM coatings are applied directly to a metal surface with the ability to adhere without the need for extensive cleaning or pretreatment. Ideally these coatings can be applied in one step directly to the metal. However, DTM coatings can also be comprised of one coat of primer and one coat of topcoat applied over metal surfaces that are properly prepared to eliminate surface contaminants and oxides. The primary advantage of DTM coatings is that they do not require a multistep operation of cleaning, pretreatment and sealing prior to painting. Current DTM technologies include solvent borne, waterborne and high solids. They can be one- or two-component acrylic, epoxy or polyurethane, or comprised of unsaturated polymers/oligomers that cure through polymerization.
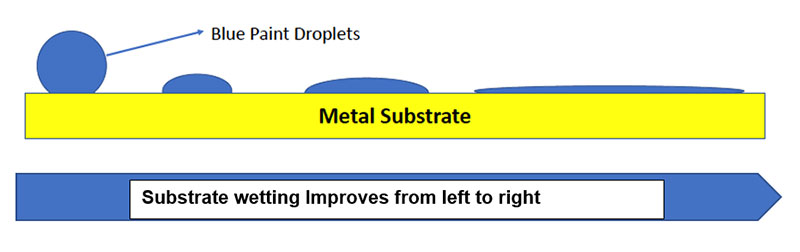
There are multiple issues to consider in designing a DTM coating that provides longer term performance. These include:
- Wetting of the substrate
- Initial adhesion
- Longer term adhesion and corrosion resistance
Wetting of the substrate
Wetting of the metal surface is a major factor that effects initial adhesion. If the coating does not readily spread or wet the surface, adhesion will be adversely effected. Stating this in a another way–the surface tension of the substrate must be higher than that of the applied coating to ensure good flow and leveling. In the diagram above, the blue sphere represents a paint droplet, and the yellow line represents a metal surface. The droplet on the right completely wets the metal surface thus providing the best opportunity to provide adhesion.
There are two ways to ensure good substrate wetting. From a substrate standpoint, the first is to increase the surface area of the substrate–for example, through abrasion and/or sandblasting. This process also removes the metal oxide and hydroxide layer to provide a surface more amenable to forming a longer lasting surface bond. The second way is to modify the coating to ensure good wetting (e.g. lower surface tension) through the addition of suitable wetting agents as well as solvents or co-solvents which may depress the surface tension.
Once adequate initial wetting is achieved, the second consideration is reviewing the factors that contribute to initial metal adhesion.
Need help finding direct to metal coatings?
Prospector can help speed along your research with technical datasheets and access to global equipment suppliers.
Search direct to metal coatings here
Initial adhesion
Initial adhesion may be defined as the quality of adhesion to the substrate surface after the paint is cured, but prior to exposure to natural weathering and/or accelerated testing. Initial adhesion of the cured film can be quantified by such tests as ASTM D3359 Cross Hatch Tape Adhesion and/or ASTM D 4541 Pull-off Strength of Coatings that quantifies adhesion in pounds per square inch. Some considerations to enhance initial adhesion after volatiles have vaporized from the paint film include:
- Resin systems with functional groups that promote bonding to the metal surface
- The presence of suitable adhesion promoters and coupling agents
- The number and type of crosslinks
Resin systems with functional groups
Resin and crosslinker systems with the ability to form hydrogen bonds or covalent bonds with the layer of oxide and hydroxide on the metal surface normally provide the best initial adhesion. Long-term adhesion and corrosion protection depends on the resin backbone and crosslinking.
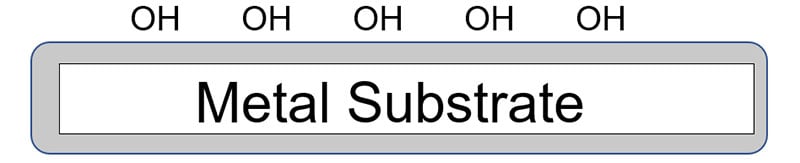
The presence of suitable adhesion promoters and coupling agents
To promote adhesion, resins and crosslinkers that contain a plethora of active hydrogen donor and accepting groups should be used. Such resins contain one or more of the following functional groups:
- carboxyl (hydrogen donating group)
- amine (hydrogen accepting group)
- hydroxyl
- amide
- urethane
- phosphate (all hydrogen accepting or donating)
The number and type of crosslinks
Accordingly it makes sense why epoxies crosslinked with amino-amide groups (hydroxy, ether, amino and amide functional groups), polyurethanes and polyureas (from moisture cure urethanes for example) provide excellent adhesion to metal surfaces. Thus, they are used widely in direct to metal applications.
The addition of a suitable silane coupling agent also can enhance both initial and long-term adhesion properties. A coupling agent is a molecule that is comprised of a reactive group on one end of the molecule ( Y ) for reacting with a functional group on the polymer chain with the other end of the coupling agent ( – Si – OR3 ) reacting with the metal surface.
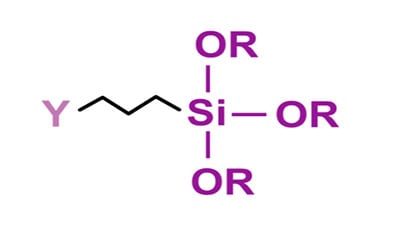
In the above molecule, the -OR groups attached to silicon can be methoxy or ethoxy, where the Y portion of the molecule is a functional group such as amino, epoxy, isocyanate, methacrylate or vinyl. The reaction involves first hydrolysis of the alkoxy group to form a silanol which undergoes a further reaction with the hydroxyl groups on the metal surface. The other end, or Y portion, of the coupling agent reacts with a functional group on the resin backbone.
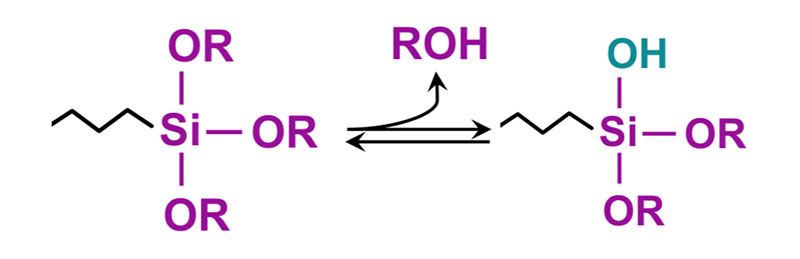
Table I- Examples of trialkoxy organofunctionalsilanes and their application
| R = Reactive Group onR-Si (-OCH3) or R-Si (-OCH2CH3) | R group Reacts with | Reactive SilaneExample | Trialkoxy Silane Reaction | Application |
| Amino | Epoxy functionality | 3-aminopropyl-triethoxysilane | With –OH on surface as well as self-crosslink to form– Si – O – Si – | Coatings for glass as well as oxides of Al, Zr, Sn, Ti and Ni |
| Epoxy | Amino functionality | 3-glycidyloxypropyl trimethoxysilane | With –OH on surface as well as self-crosslink to form– Si – O – Si – | Coatings for glass as well as oxides of Al, Zr, Sn, Ti and Ni |
| Meth–acrylate | Acrylic resin polymerization | 3-methacryloxypropyltrimethoxysilane | Self-crosslink with another silane to form– Si- O – Si – and with –OH on the surface | Moisture cure resins with improved adhesion, physical and environmental performance |
| N/A | N/A | N-octyltriethoxysilane | Forms– Si – O – Si – | Water repellency, improved hydrophobicity |
| Vinyl | Vinyl or acrylic resin polymerization | Vinyl-trimethoxysilane | Forms– Si – O – Si – | Moisture cure resins with improved adhesion and film integrity. Also used as a moisture scavenger |
| Isocyanate | Hydroxyl, Amino or Mercapto | 3-isocyanatopropyl-triethoxysilane | With –OH on surface as well as self-crosslink to form– Si – O – Si | Coatings for metallic and inorganic oxides, also moisture cures |
| SilaneSIVO Sol-Gel | VOC Free Waterborne Surface Treatment for various metals and surfaces |
Longer term adhesion and corrosion resistance
Lastly, to provide longer term adhesion and corrosion protection, the DTM primer should be formulated with a quality resin system, contain corrosion inhibitive pigment(s) and resist moisture penetration. The latter quality can be accomplished by increasing hydrophobicity and crosslink density. A long lasting moisture resistant primer also has the ability to resist hydrolysis of the cured film.
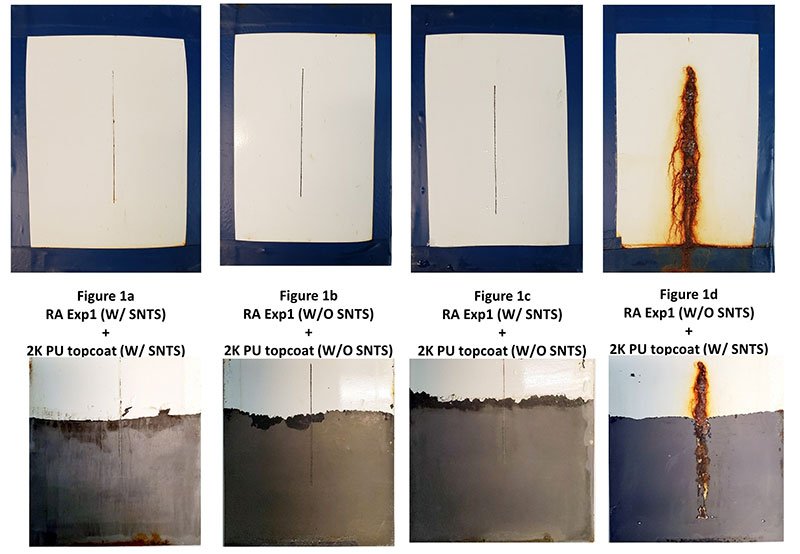
Figure 2 illustrates the type of corrosion protection that can be achieved with a formulation that provides excellent substrate wetting, superb initial adhesion, long-term corrosion resistance and high hydrophobicity.
Figure 2. Rust Armour primer with a two component urethane topcoat formulated by Chemical Dynamics–utilizing a high crosslinking resin system with and without combinations of hydrophobic pigment modification (SNTS).
10,000 ASTM B117 Salt Spray of Properly Formulated Direct to Metal 2 Coat Paint System (bottom row represents paint film removed).
Long term corrosion resistance is an important consideration along with the selection of a resin/coating system that provides wet adhesion and minimizes the penetration of moisture and oxygen. As resin Tg and cross-link density increases, moisture and oxygen penetration decreases. In addition, low permeability rates help to provide wet adhesion as less water will desorb when the coating is removed from its service environment. Resins with a high amount of aromatic character (bisphenol A based epoxies, polycarbonate and styrenated resins) have low oxygen permeability. Halogenated resins such as vinyl chloride, copolymers, chlorinated rubber and fluorinated polymers such as poly (vinylidene fluoride) all have low water solubility and thus low moisture permeability rates1 (see Table II).
In summary, the formulation of DTM coatings to deter corrosion is a complex undertaking and depends on the metal substrate, service environment, pigment level and type of resin selection. For additional information concerning resin and material selection to formulate corrosion inhibitive coatings, please navigate to www.ulprospector.com.
Sources:
- www.faybutler.com/pdf_files/HowHoseMaterialsAffectGas3, Welding Journal.
References:
Prospector Knowledge Center and Search Engine
Zeno W. Wicks Jr., Frank N. Jones, Socrates Peter Pappas, Douglas A. Wicks. (2007). Organic Coatings: Science and Technology, Third Edition.
Wiley, Jones e.al. (2017) Organic Coatings, Science and Technology, Fourth Edition.