Silicate coatings are alkali metal silicates that are made from naturally occurring materials such as sand and alkali. Alkali metal silicates are derived from a combination of silica (SiO2) and a carbonate of lithium, sodium or potassium to produce a silicate (SiO2/Na2O). Depending on their formulation, these remarkable coatings can have multiple benefits including:
- Not petroleum based
- Outstanding durability
- UV resistant
- Acid rain resistant
- High hardness
- Exceptional wear resistance
- Outstanding hardness
- Non-flammable
- Adhere to multiple substrates
- High moisture and gas permeability (can be a benefit or a disadvantage)
- Chemically bond to mineral surfaces
- Heat Resistance (most silicate based paints have a softening point of ~ 1,200F)
- Heat Resistant paint for metals (silicates mixed with copper, nickel, chromium or stainless steel powders)
- Good chemical and physical properties
- Zero VOC
Research coating materials
Did you know Prospector gives you access to technical data on thousands of paints and coatings materials, as well as the opportunity to request samples and contact global suppliers?
Create your free account now!
Types of silicate-based coatings
Types of silicate-based coatings include silicate, silicate-organic emulsion and lastly sol-silicate.

Soluble silicates include those of the Group 1A elements of the Periodic Table (Li, Na and K). As Silicates are based on alkali metal oxides and silica, their solutions are alkaline. As the molar weight ratio of the silicon:alkali metal increases, the pH decreases:
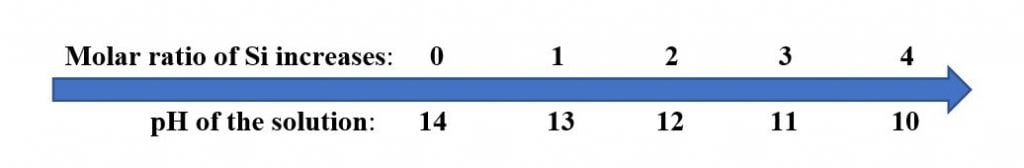
Accordingly, when blending alkali metal silicates with organic emulsions, it is important to use higher ratios of silicon to alkali metal to achieve the best stability and a workable pH of 8 – 10 for most organic-based emulsions.
Viscosity of sodium silicate solutions is a function of concentration, density and ratio of sodium: silicon. Higher or lower ratios increase viscosity with a minimum viscosity reached at a 2.0 weight ratio.
From a structural standpoint, waterborne silicates are glasses that have a wide variety of molecular structures in which the anions are monomers, dimers, trimers, branched chains, and ring structures, as well as other three dimensional networks. Cations of alkali metals (Li+, Na+ and K+) attach to the anions (Si – O – ) to create a complex alkali silicate.
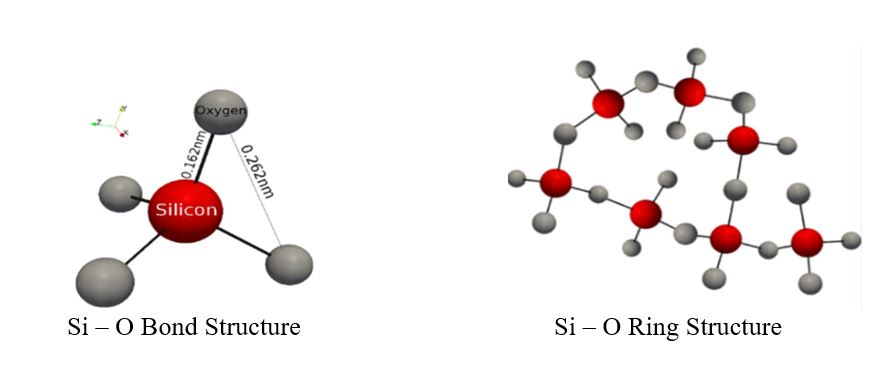
There are two equilibria in an alkali silicate solution, that includes an acid-base equilibrium:
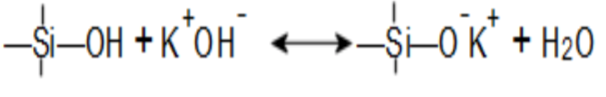
As well as a condensation polymerization-depolymerization equilibrium:
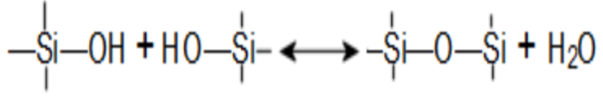
Irreversible reactions also take place with polyvalent cations such as Ca++ or may also include Mg++, Fe, or Mn.
The ratio of alkali metal oxide to silica has a significant effect on coating properties as illustrated in the table below:
| The higher ratio (High SiO2 low NaCO3, e.g. 3.75 to 1) gives: | The lower ratio (Low SiO2 High NaCO3, e.g. 2 to 1) gives: |
| Lower viscosity | Higher specific weight |
| Faster drying speed | Greater solubility |
| Faster curing speed | Higher pH value |
| Increased susceptibility to low temperatures | Greater susceptibility to water influence |
| Higher chemical resistance of coatings | Higher tack and binding power |
Commercially available silicates are normally produced in ratios of 1.5 or higher. Coatings based on sodium silicate can be used and require a catalyst for ambient cure, but are susceptible to efflorescence. Solutions of sodium silicate can react or cure with dissolved polyvalent ions including Ca++, Al+++ and Mg++ to form insoluble silicates.
- Potassium silicates are self-curing, however the reaction is slow.
- Lithium silicates have low water solubility and are used to minimize water soluble by-products and efflorescence.
- Efflorescence is a whitish, powdery deposit on the surface of a material (stone, concrete, brick and mortar) caused from mineral-rich water percolating to the surface through capillary action. Efflorescence usually consists of gypsum, salt, or calcite.
Mineral calcium carbonates (e.g. calcite) exhibit low reactivity with soluble silicate, whereas precipitated calcium carbonate provides high reactivity. The viscosity of sodium silicates is very high, whereas colloidal silicas (stabilized silica particles less than < 100nm in size) have viscosities closer to that of water. pH has a major impact on the viscosity of colloidal silicas and form gels at a pH < 7 and a Sol when a pH is >7. Liquid sodium and potassium silicates also can be reacted with a variety of acidic or heavy metal compounds to produce solid, insoluble bonds or films.
Neutralizing an alkali silicate with acidic materials (e.g., aluminum sulfate) polymerizes the silica and forms a gel. This produces a bond or film on surfaces where gellation occurs. Chemical setting agents that can be used in this manner include: mineral and organic acids, carbon dioxide (CO2) gas, and acid salts such as sodium bicarbonate and monosodium phosphate (NaH2PO4).
Silicate-emulsion paints comprise a low level of a polymeric organic emulsion (~5%) with an alkali silicate. The emulsion helps to enhance water resistance until the silification reaction is complete, which can take weeks. Higher levels of organic emulsions are generally incompatible.
Typical components of a silicate-emulsion paint can include:
- organic additives like compatible surfactants
- small amounts of suitable coalescing solvents
- thickeners (e.g. Hydroxyethylcellulose, or HEC), stabilizers and modifiers
- emulsions that are stable at higher pH that may include:
- aqueous dispersions of polymers such as:
- styrene-butadiene
- polystyrene
- neoprene
- polyvinyl chloride
- polyvinyl acetate
- acrylonitrile copolymers
- acrylic polymers and copolymers
- inorganic binders such as potassium silicate and filler pigment
- inorganic alkali resistant pigments
- aqueous dispersions of polymers such as:
As silicate paints are not generally flexible, they can be flexibilized by the addition of 1 to 5% by weight of glycerine or other polyhydric alcohols. Up to 30% of sorbitol can be used, provided the silicate solution is diluted to avoid excessive thickening.
Rubber lattices can also be employed as plasticizers. Incorporation of finely ground clays and similar fillers will improve flexibility to some extent. Silicate emulsions paints can also be formulated for use on aluminum, galvanized steel, steel, stone, brick, concrete, and previously painted surfaces that used an emulsion paint.
Sol-silicate paint is a combination of silica-sol and potassium silicate. An organic binder is incorporated at a percentage of 10% or lower. As opposed to most other silicate paints, sol-silicate paints bond to non-mineral substrates through both physical and chemical bonds. Silica sols are dilute solutions of dissolved silica that are at an acidic pH.
Sources and further reading:
- Prospector Knowledge Center and Search Engine
- Bulletin 12-31, PQ Corporation, Bonding and Coating Applications of PQ soluble Silicates
- Book One. Waterborne Silicate Coatings, Rev 3, July 2016, Ken Marx