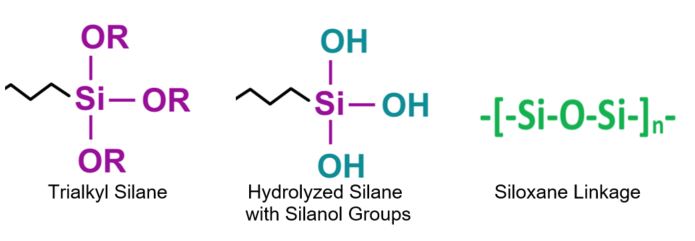
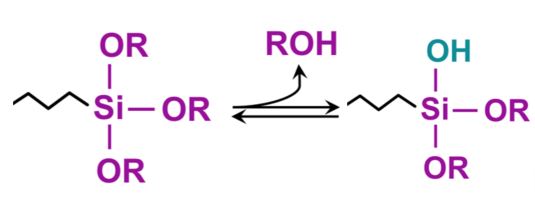
Coated surfaces can impart a wide range of affinity related to water, from hydrophilic (water loving), to hydrophobic (water repelling) to superhydrophobic (super water repellency). These surface characteristics are obtained by the proper combination of surface morphology at the micro and/or the nanoscale level, combined with a low surface energy material.
Superhydrophobicity and the lotus leaf
A prime example of superhydrophobicity in nature is the lotus leaf. The lotus leaf has a microstructure comprising small protuberances or spiked papillae 10 – 20 microns in height and 10 – 15 microns in width which have a second hydrophobic wax layer. The combination of a structured surface combined with a low energy wax provides superhydrophobicity to the surface. To fully explain and quantify hydrophobicity, it is necessary to define the relationship between contact angle and the hydrophobic/hydrophilic character of a surface.
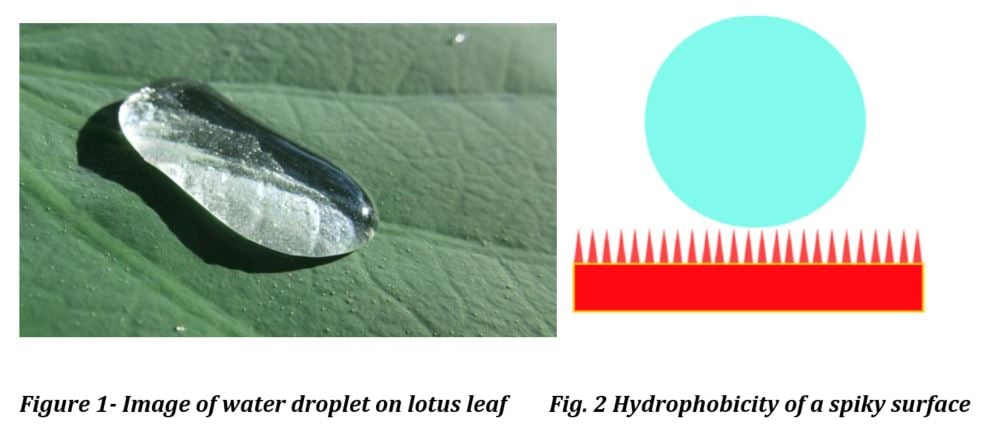
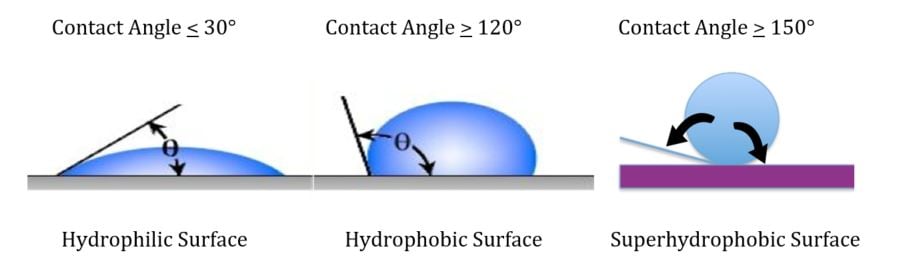
Contact angles of 150° or more and are called superhydrophobic – meaning that only two to three perfect of the surface of a water droplet is in contact with the surface. Since the surface contact area is less than 0.6 percent, this provides a self-cleaning effect. The ramifications of imparting lotus leaf water repellency characteristics to a coating surface has profound performance implications which can include the following:
- Self-Cleaning – Contaminants that fall on a superhydrophobic/hydrophobic surface are removed as water droplets will roll off.
- Improved moisture resistance – Improved blister resistance and gloss retention
- Improved corrosion resistance – Lowering moisture penetration reduces or even eliminates water and soluble salt penetration to the metal substrate which greatly slows the onset of corrosion.
- Extended life cycle for coating and substrate – Increased coating weatherability and resistance to the penetration of soluble salts and moisture positively impacts the longevity of the coated article.

The role of surface tension
We have discussed the role that surface morphology plays in imparting hydrophobicity; the other critical component for hydrophobicity is surface energy.
- Surface tension is the elastic tendency of liquids that make them acquire the least surface area possible.
- Surface tension is measured along a line, whereas surface energy is measured along an area.
Components of surface tension mainly include dispersive and polar, hydrogen bonding and acid-base contributions. In general lower surface energy materials provide higher hydrophobicity. Table 1 and 3 lists the Surface Free Energy of several polymer types and modifiers, respectively, used in coatings, whereas Table 2 provides surface tensions of commonly used solvents in coatings.
| Polymer | Surface Free Energy mN/m |
| Polyhexafluoropropylene | 12.4 |
| PTFE | 19.1 |
| PDMS | 19.8 |
| Parafin Wax | 26.0 |
| Polychlorotrifluoroethylene | 30.9 |
| Polyethylene | 32.4 |
| Polyvinyl Acetate | 36.5 |
| Polymethylmethacrylate | 40.2 |
| Polystyrene | 40.6 |
| Polyvinyldene Chloride | 41.5 |
| Polyester | 43 – 45 |
| Polyethyleneterephthalate | 45.5 |
| Epoxypolyamide | 46.2 |
Table 1 – Surface Free Energy of Polymers
| Solvent | Surface TensionDynes/cm |
| Water | 72.8 |
| Toluene | 28.4 |
| Isopropanol | 23.0 |
| n-Butanol | 24.8 |
| Acetone | 25.2 |
| Methyl propyl ketone | 26.6 |
| Methyl amyl ketone | 26.1 |
| PM acetate | 28.5 |
Table 2 – Surface Tension of Solvents
| Material Identity | Critical Surface TensionmN/m |
| Heneicosafluoro-dodecyltrichlorosilane | 6-7 |
| Heptadecafluorohexyl--trimethoxy Silane | 12.0 |
| PDMS | 19.8 |
| Octadecyltrichlorosilane | 20-24 |
| Nonafluorohexyl-trimethoxysilane | 23 |
Table 3 – Surface Free Energy of Potential Surface Modifying Agents
When two different liquid materials are applied to a solid surface, the liquid with the lower surface tension will flow or wet out on the solid surface, for example polyethylene, more so than the liquid with the higher surface tension. For example, water (surface tension 72.8 Dynes/cm) will form a higher contact angle than will Toluene (surface tension 28.4 Dynes/cm).
Thus far, we’ve defined the factors that contribute to the hydrophobicity, or the lack thereof, including contact angle, surface structure, and why most organic solvents tend to wet a surface better than water as a consequence of their lower surface tension. The next segment will concentrate on how to impart greater hydrophobicity to a coating system, especially from a surface perspective.
Maximizing surface hydrophobicity
To maximize the surface hydrophobicity of a coating, the surface energy should be as low as possible. A low surface energy, coupled with an appropriately structured surface, maximizes hydrophobicity.
Surface energy has the same units as surface tension (force per unit length or dynes/cm). A high surface tension liquid such as water will have maximum hydrophobicity and thus have poor wetting (high contact angle) over a coating surface that has a lowsurface energy. As Table II illustrates, surface energy can vary greatly depending on the nature of the surface that comes in contact with water.
For instance, a coating surface that is rich in polydimethylsiloxane (Surface Energy 19.8 mN/m) at the surface will provide a more hydrophobic surface than that of polystyrene (40.6 mN/m). In general terms, to provide the greatest hydrophobicity, the material’s most hydrophobic moiety should be positioned on the surface.
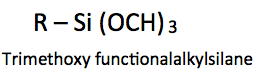
As another example, if an organofunctional trimethoxysilane is used for surface modification, the methoxysilane groups should be engineered to be positioned at the surface. Perfluoro and aliphatic groups at the coating surface offer greater hydrophobicity than that of ester or alcohol groups. Ester and alcohol groups are more polar in nature and thus more receptive to water deposited on the surface. For example, from lowest to highest surface tension:

Providing increased hydrophobicity throughout a properly engineered coating can provide additional attributes such as self-cleaning, improved corrosion and moisture resistance and an extended life cycle for the coating and substrate.
Recent advances in silane technology have enabled the availability of silanes for use in waterborne systems for improved hydrophobicity. Accordingly, resin selection, flattener, extender pigments and opacifier pigments can also be selected to maximize hydrophobicity.
Secondly, formulations utilizing nanoparticles must be tailored to provide proper acceptance rather than as a drop-in to achieve a desired property.
Search UL Prospector® for hydrophobic raw materials.
You might also be interested in…
- Surface Tension & Surface Energy
- Breaking the tension with surfactants [INFOGRAPHIC]
- Hydrophobic Coatings Explained
- Dispersing & Wetting Hydrophobic Pigments & Fillers in Water-Based Paints to Avoid Pigment Flooding & Floating
Sources
- Prospector Knowledge Center: Hydrophobic Coatings Explained
- Gelest 2016 product literature