Electrically-conductive particles for use in coatings have taken a quantum leap forward in recent years. Revolutionary materials like carbon nanotubes (CNTs) and graphene possess 200 times greater strength than steel, with conductivity better than copper. This unique combination of strength, conductivity and high temperature resistance have the promise to impart exceptional properties in coatings that challenge the imagination. This article will discuss and contrast conductive materials with an emphasis on conductive nanomaterials and their potential advantage in coatings applications.
More than 200 conductive materials
That’s what you’ll find in Prospector®. Conductive additives, resins, pigments…Find materials faster with Prospector.
Click here to search for conductive coatings materials now
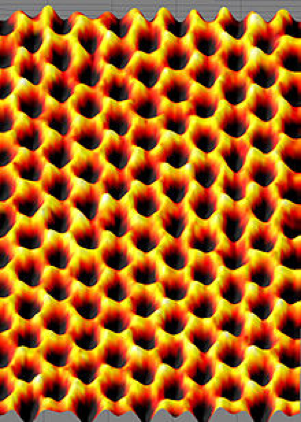
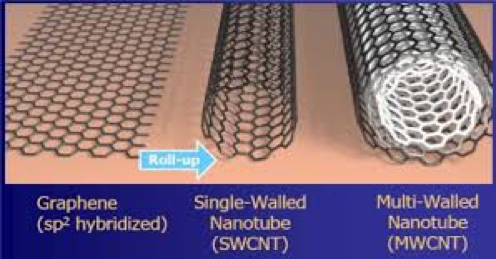
Other more commonly used electrically-conductive particles include conductive carbon black, graphite, quaternary ammonium salts, copper, aluminum, silver and combinations thereof. In addition to conductive particles, there are a number of conductive polymers that can provide conductive coatings that may be discussed in a future article. Surface resistivity of coatings is nearly independent of relative humidity. Figure 1 illustrates the structure of graphene, graphene is a two-dimensional structure and can be thought of as a single walled carbon nanotube in sheet form.
| Conductivity = S/cm or S/mSurface resistivity = Ω/ (ohms per square)Surface resistance = ΩVolume resistivity = Ω x cmVolume (bulk) resistivity = (Resistivity x length x width) / height |
| Volume resistivity is a measure of sheet resistivity across defined thickness |
| Surface resistivity is the most common means used to characterize conductive coatings. ASTM D257 is the most widely-used standard for measuring surface resistivity. |
| Percolation theory: As concentration of a conductive material in a coating increases, the conductivity will reach a point where the conductivity will abruptly increase once a critical concentration is reached. |
Table 1 – Common Terminology of Conductive Materials
For electric current flow across a surface, surface resistivity (ohms/square) can be defined as the ratio of voltage drop per unit length, to the surface current per unit width.
Antistatic refers to the property of a material that inhibits triboelectric charging. Antistatic coatings have a surface resistivity of at least 1 X 109, but less than 1 X 1012.
Static dissipative coatings have:
- a surface resistivity of 1 X 105 ohms/sq. or
- a volume resistivity of 1 X 104 ohm-cm, but less than 1 X 1012 ohm/sq. surface resistivity or
- 1 X 1011 ohm-cm volume resistivity.
Conductive coatings have a surface resistivity of less than 105 ohm/sq., or a volume resistivity less than 104 ohm-cm.
| Paint Film with 2% conductive particles on total weight | ASTM D257Surface ResistivityOhms/square | Considerations |
| Quaternary Ammonium salt | ~ 1013 | Water sensitivityMigration to surface |
| Conductive carbon black | ~ 1010(antistatic) | Color |
| Graphene | ~ 107(static dissipative) | ColorDispersibilty |
| Carbon nanotubes (CNTs) | < 103(conductive) | ColorDispersibility |
Table 2 – Films with conductive additives
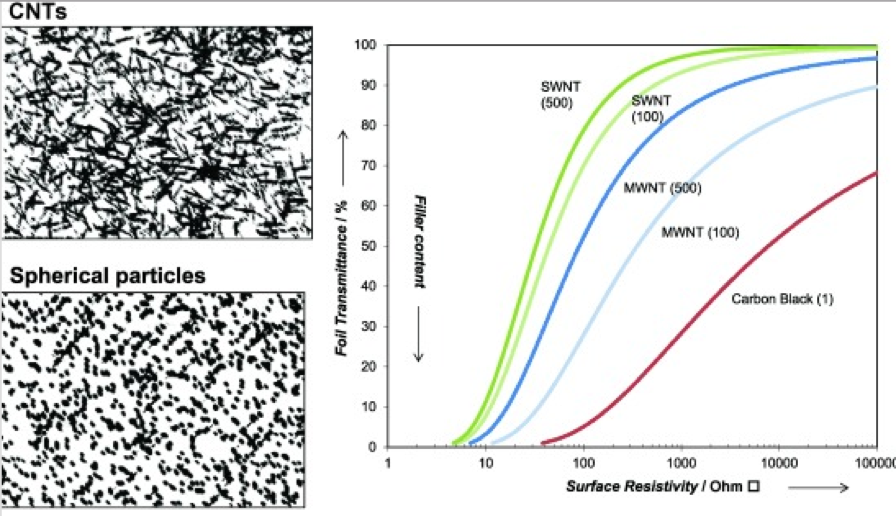
An important aspect of obtaining optimum conductivity from both graphene and CNTs for water or solvent-borne applications is to ensure that the dispersion is optimal and stabilized. If graphene and/or CNTs are not effectively dispersed, the particles will not be adequately separated and form bundles of particles. These materials tend to self-associate and form clusters, which in turn do not provide adequate connectivity to support optimum conduction of an electric current.
To overcome this issue, in many cases nonconventional means of dispersion are employed. Sonication is one such method that untangles nanoparticles, and thus provides the connectivity to support a current (percolation).
The agglomeration of graphene and CNTs is known to have a major impact on the percolation threshold and thus electrical conductivity of their nanocompositions. The interface between the conductive materials also has a profound effect on conductivity. The percolation threshold is the threshold at which the concentration of conductive nanomaterial of the composition displays an abrupt transition from insulator to a conductor. The aspect ratio of a conductive material (width:height) also plays an important contribution to the percolation threshold (lower aspect ratios favor conductivity).
The reason for the conductivity of graphene and more so CNTs is their extensive network of sp2 bonds and stacking of their pi bonds.
Graphene can be described as a single layer thickness of graphite. Graphite is also electrically conductive and available in different purities as well as amorphous or crystalline forms.
One of the other reasons why nanoparticles such as graphene and CNTs are so conductive is that they are ultrasmall. As the dimension of a particle decreases, the ratio of surface area to volume increases quite dramatically. Larger surface area produces greater interaction of particles and higher attractive forces. For example, a surface area over 100nm in size normally defeats the advantage that nanoparticles provide to enhance performance.
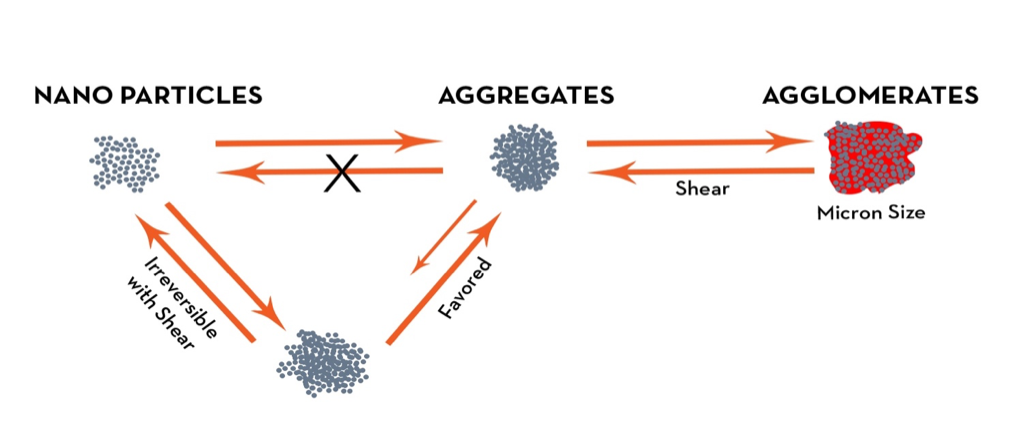
The proper use of conductive nanoparticles in coatings can impart multiple beneficial properties. Stabilization of dispersed nanoparticles is essential to optimize the full benefits of these materials. Secondly, formulations utilizing nanoparticles must be tailored to provide proper acceptance, rather than as a drop-in to achieve a desired property.
| Material | Resistance Ohm-M |
| Graphite | 1 X 10-5 |
| Brass | 0.9 X 10-7 |
| Platinum | 0.98 X 10-7 |
| Silver | 1.6 X 10-8 |
| Aluminum | 2.8 X 10-8 |
| Copper | 1.7 X 10-8 |
| Zinc | 5.5 X 10-8 |
In addition to their electrical properties, graphene, graphite and CNTs provide good stability at high temperatures. Furthermore, due to their unique molecular structure, when properly dispersed, they can also enhance mechanical properties. The temperature stability of CNTs in air is reported to be 750° C, graphene and graphite in excess of 600° C.
Applications for future coatings using the next generation of conductive materials will include:
- coatings to prevent electronic discharge for electrical applications
- communication equipment
- consumer electronics
- computer equipment
- flexible electrical equipment
- electronic applications requiring high temperature resistance and enhanced mechanical properties.
In summary, properly formulated coatings utilizing conductive particles and especially conductive nanoparticles can achieve performance attributes heretofore not obtainable by other means.
References and further reading:
- Journal of Applied Physics, August 7, 2015, Wang et.al.
- Prospector: Nanoparticles – When Smaller is Better
- ResearchGate: Nano-Bio-Technology and Sensing Chips: New Systems for Detection in Personalized Therapies and Cell Biology
- ResearchGate: ChemInform Abstract: Carbon Nanotubes: An Example of Multiscale Development – A Mechanistic View from the Subnanometer to the Meter Scale
