Silanes were first discovered and identified in 1857 by German chemists Heinrich Buff and Friedrich Woehler among the products formed by the action of hydrochloric acid on aluminum silicide.1 Since that time silane chemistry has proven to be a versatile means to enhance performance of organic-based coatings, or to provide siloxane-modified coating systems with a variety of performance characteristics not readily achievable with other technologies.
Interested in using an organosilane in your next formulation?
UL Prospector has listing for a variety of materials from global suppliers. Find technical data, request samples, and contact suppliers directly – all right within Prospector!
Search organosilane materials now
Depending on the proper selection of reactive silane, a variety of improved performance attributes can result, including:
- Weathering
- Adhesion
- Hardness
- Flexibility
- Moisture resistance
- Lubricity
- Cross-link density
- Corrosion resistance
Silane and Siloxane Structures:
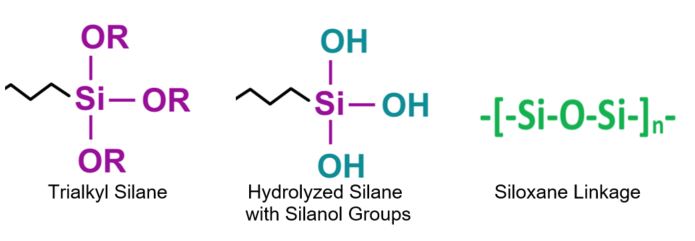
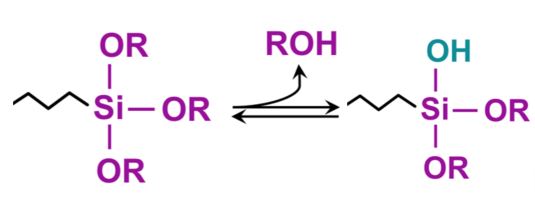
In the presence of water, a trialkoxysilane can hydrolyze as a first step in the reaction to liberate methanol (for a trimethoxysilane) or ethanol (for a triethoxysilane) and self-condense to form a siloxane or react with available alcohol groups on a pigment, polymer or substrate to provide a siloxane linkage.
Silanes are used in a number of applications to:
- Improve adhesion to inorganic or organic surfaces – Silanes, when added to paints, can enhance adhesion to inorganic surfaces including metals and glass
- Coupling Agents – Silanes are used for coupling organic polymers to inorganic materials including pigments and fillers
- Crosslinking Agent – Selective organofunctional alkoxysilanes can react with organic polymers to provide a trialkoxysilyl group into the polymer backbone. In turn, the silane can then react with moisture to crosslink and form a three-dimensional siloxane cross-linked structure.
- Dispersing Agent – Used to increase the hydrophobicity of inorganic pigments and improve flow characteristics and the ability to be dispersed in organic polymers and solvents.
- Improved hydrophobicity – Selective reactive silanes can be modified to provide superb hydrophobicity (to be discussed more in the sequel to this article)
- Moisture Scavenger – In moisture sensitive formulations, the three alkoxysilane groups can scavenge water by reacting with moisture to form alcohol molecules.
- Pretreatment for metal surfaces – Specialized waterborne silanes for pretreatment of various metal surfaces (e.g. Evonik’s Dynasylan SIVO product group)
A silane that contains at least one carbon silicon bond (CH3 – Si -) is called an organosilane. Reactive silane is the term used to define compounds that have a trialkoxysilyl group and an alkyl group (R) containing a reactive constituent.
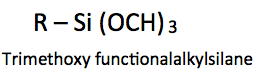
Trialkoxysilyl groups can react directly, or indirectly in the presence of water with hydroxyl groups. As illustrated in Table 1, the other organofunctional group (R) can participate via a crosslinking reaction with another reactive site in a coating.
In regard to the reactions and interactions with a surface, there are many complexities and dependent variables. For example, the rate of hydrolysis of the trialkoxysilyl groups with moisture to form silanol groups (R – Si- OH), which in turn self-condense or crosslink compete with the reaction of the silanol groups with the substrate hydroxyl groups. These competing reactions can vary depending on moisture level, pH, and rates of reverse reactions. as hydrolysis is reversible. Hydrolysis of trialkoxysilyl groups to silanols and the subsequent self-condensation to form a siloxy crosslink (- Si – O – Si -) can be accelerated by the use of a suitable tin catalyst such as dibutyltin dilaurate.
On the other hand, the best catalyst for promoting co-condensation between a resin and -the silicone intermediate are titanate-based catalysts such as tetraisopropyl titanate.
Except for those applications requiring polymerization of a reactive silane into a resin backbone, most of the reactions illustrated in Table I can occur under ambient conditions.
| R = Reactive Group onR-Si (-OCH3) or R-Si (-OCH2CH3) | R group Reacts with | Reactive SilaneExample | Trialkoxy Silane Reaction | Application |
| Amino | Epoxy functionality | 3-aminopropyl-triethoxysilane | With –OH on surface as well as self-crosslink to form– Si – O – Si – | Coatings for glass as well as oxides of Al, Zr, Sn, Ti and Ni |
| Epoxy | Amino functionality | 3-glycidyloxypropyl trimethoxysilane | With –OH on surface as well as self-crosslink to form– Si – O – Si – | Coatings for glass as well as oxides of Al, Zr, Sn, Ti and Ni |
| Meth–acrylate | Acrylic resin polymerization | 3-methacryloxypropyltrimethoxysilane | Self-crosslink with another silane to form– Si- O – Si – and with –OH on the surface | Moisture cure resins with improved adhesion, physical and environmental performance |
| N/A | N/A | N-octyltriethoxysilane | Forms– Si – O – Si – | Water repellency, improved hydrophobicity |
| Vinyl | Vinyl or acrylic resin polymerization | Vinyl-trimethoxysilane | Forms– Si – O – Si – | Moisture cure resins with improved adhesion and film integrity. Also used as a moisture scavenger |
| Isocyanate | Hydroxyl, Amino or Mercapto | 3-isocyanatopropyl-triethoxysilane | With –OH on surface as well as self-crosslink to form – Si – O – Si | Coatings for metallic and inorganic oxides, also moisture cures |
| SilaneSIVO Sol-Gel | VOC Free Waterborne Surface Treatment for various metals and surfaces |
Table I: Reactions of Trialkyloxy Organofunctionalsilanes and Their Applications
Reactive silanes provide utility to improve coating performance in a number of applications, including:
- Pigment wetting
- Improving hydrophobicity and increasing contact angle
- Enhancing adhesion over a number of metallic and inorganic surfaces
- Coupling agent between differential materials
- Scavenging moisture to provide improved stability
- Crosslinking to improve physical and environmental properties
A variety of siloxane-based reactive trimethoxy silane prepolymers are also available with functional groups including acylate, isocyanate, amino, hydroxyl, epoxy and vinyl. These enable a variety of opportunities to improve cross-link density, adhesion, weather resistance, moisture resistance, hydrophobicity and chemical resistance.
Resources
- Wikipedia: Silane
- UL Prospector
- Evonik, ACS Product presentation
- Organic Coatings, Science and Technology, 3rd Edition
