Polyurethanes coatings have come a long way since their invention by Otto Bayer and coworkers in 1937. Depending on the choice of oligomeric and polymeric materials, these paints are used in a variety of demanding high performance applications due to their versatility. They can be hard or soft, flexible or rigid, resistant to chemicals and provide excellent adhesion.
Polyurethane properties and applications
- Outstanding moisture and corrosion resistance
- Flexible primers and topcoats
- Weather resistance (aliphatic polyisocyanate with suitable durable polyol)
- Resistance to acid rain and other chemicals
- One component
- Two component
- Waterborne one component bake finishes
- 100% solids
- Powder coatings
- Waterborne ambient cure two component finishes
Polymeric and isocyanate prepolymer components include one or more isocyanate prepolymers and one or more polymeric or oligomeric components containing hydroxy functionality or other active hydrogen group. Isocyanates are reactive with functionalities which include:
- Hydroxy
- Amino
- Imino
- Ketimene
- Carboxyl (forms CO2)
- Urethanes
- Ureas
- Acetoacetylated resins
The active hydrogen for exterior weatherable coatings is normally an aliphatic hydroxyl group in a polyester or acrylic polymer. Alcohols and phenols react with an isocyanate to form urethanes.
Need help finding polyurethane coatings?
Prospector can help speed along your research with technical datasheets and access to global equipment suppliers.
Search polyurethane coatings here
Urethane reactions
In the following reaction, R1 and R2 can be aliphatic or aromatic.
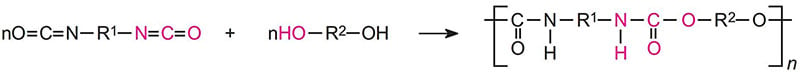
The urethane reaction is reversible at higher temperatures. For baking systems such as those using blocked isocyanates, excessive bake temperature can result in embrittlement, color change and a decrease in moisture and corrosion resistance.
As a general rule, reaction rates for urethane formation is in the following order:
primary hydroxyl > secondary hydroxyl > tertiary hydroxyl. The reverse reaction rate is the inverse of the forward reaction. For example urethanes from tertiary hydroxyls are relatively unstable.
Once formed, urethanes can react further with isocyanates to form allophanates:
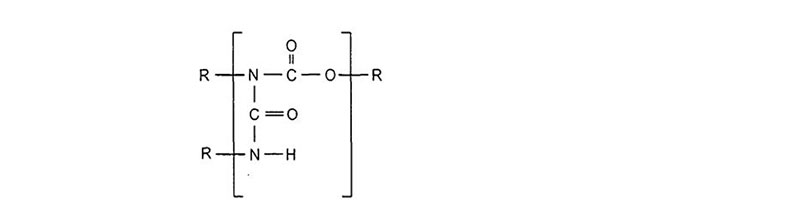
Other ambient cure reactions of an isocyanate and polyol follow:
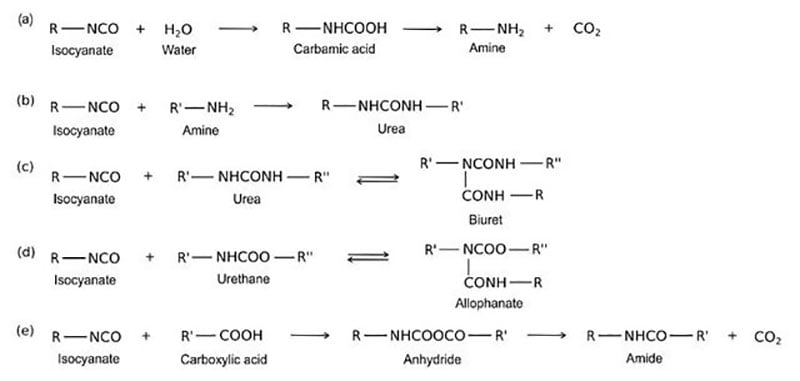
As illustrated above, the desired crosslinking reaction between a polyol and an isocyanate to form a polyurethane involves multiple competing reactions. For this reason, two-component formulations with polyol in one component and isocyanate in a second component are normally formulated with a 10% or more stoichiometric excess of isocyanate to overcome competing reactions with moisture and other possible reactants.
Polyurethane catalysts
Catalysts for polyurethanes include tin based carboxylates such as dibutyl tin dilaurate, dibutyl tin octoate or tertiary amines such a DABCO [N2(C2H4)3]. For toxicity concerns, there are also tin-free catalysts based on bismuth neodecanoate, bismuth 2-ethylhexanoate or other metal carboxylates.
Isocyanates and polyisocyanates
There are multiple aliphatic and aromatic polyisocyanates available for use in ambient cure two-component solvent born, 100% solid liquid or powder, as well as waterborne paints. Blocked isocyanates are used in single component baked coatings as they unblock at an elevated temperature to activate the isocyanate group. The reaction sequence is first unblocking and then addition. Polyurethanes formed from aromatic isocyanates are used primarily in primers and interior coatings due to poor light stability, but excellent moisture and corrosion resistance.
Common aliphatic and aromatic polyisocyanate building blocks include:
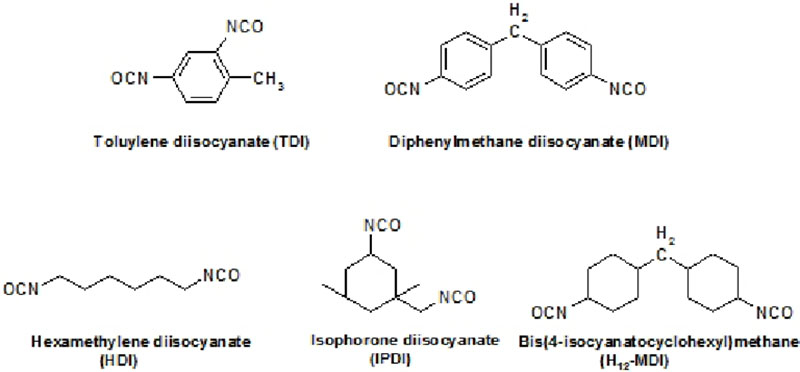
HDI and IPDI are used to synthesize higher molecular weight isocyanate prepolymers which may include isocyanurates, allophanates and uretdiones to improve hygiene, handling and weathering properties.
Isocyanates can be blocked to form a stable material for use as a crosslinker in single component polyurethane coatings. Blocked isocyanates are used extensively in powder, waterborne and high solids baking finishes for coil primers, automotive coatings and electrodeposition coatings. Common blocking agents include 2-ethylhexanol, e-caprolactone, methyl ethyl ketoxime and 2-butoxy ethanol. When mixed with a polyol, blocked isocyanates are stable until they reach the unblocking temperature and then eliminate the blocking agent and react with the polyol to form a polyurethane.
Waterborne two component urethane coatings can be made using water dispersible isocyanates. Water dispersible IPDI or HDI based isocyanates are commercially available and are made by reacting a portion of the isocyanate groups with polyethylene glycol monoether. The polyisocyanate is then added into a separate dispersion containing the polyol to form separate dispersed particles that crosslink and form a film.
Iso-free technology
Isocyanate-based technology has come under increased scrutiny as exposure to isocyanates can cause asthma and other respiratory issues. Occupational asthma has overtaken asbestosis as the leading cause of new work-related lung disease. Isophorone free technology provides polyurethane formation without exposure to free isocyanate. In the last few years isofreetechnologies have emerged that do not utilize isocyanate crosslinkers to form a polyurethane and thus eliminate isocyanate exposure. Isofree 2K technology utilizing polycarbonate and polyaldehyde for example includes improved sprayable pot life and rapid cure and early hardness. Technologies that form polyurethanes without the use of an isocyanate crosslinker follow:
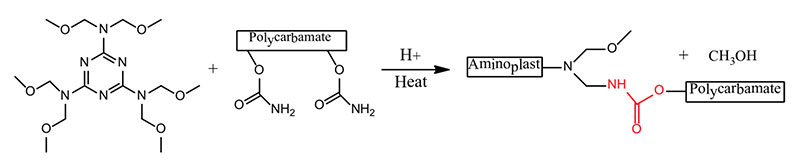
- Hexamethoxy methyl melamine + Polycarbonate → Polyurethane
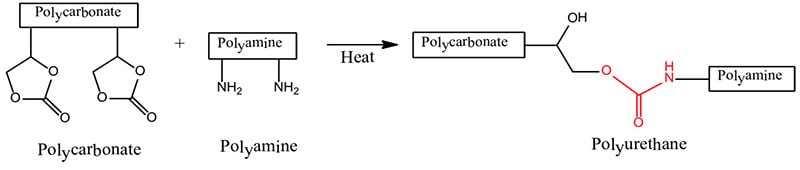
- Polycarbonate + Polyamine → Polyurethane
- Polycarbamate + Polyaldehyde → Polyurethane
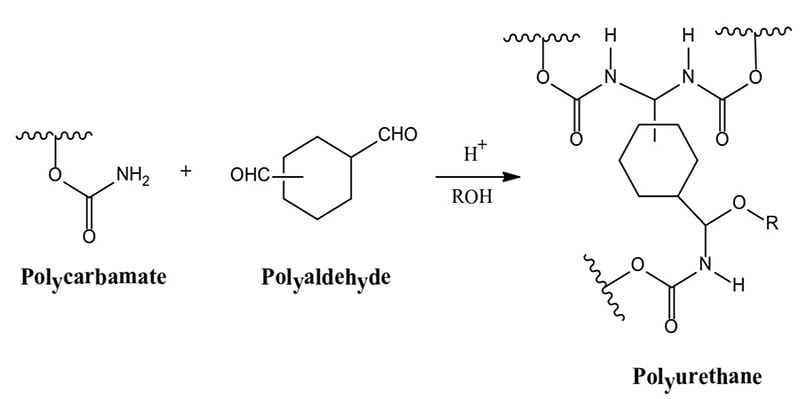
The formation of polyurethanes in reactions #1 and #2 are sluggish at room temperature, whereas the reaction rate of #3 that utilizes the crosslinking reaction of a polycarbonate and a polyaldehyde is more facile. Polyurethane formation by this reaction route provides a longer sprayable pot life and at the same time a faster reaction rate after application than that provided by the use of an isocyanate crosslinker.
Sources:
Prospector Knowledge Center and Search Engine
Polyurethanes. (2017). The Essential Chemistry Industry – online.
Mahendra, Vidhura. (2016). Foam making via pine resins. 10.13140/RG.2.1.2065.0004.
Wikepedia. Polyurethane.
John Argyropoulos, Nahrain Kamber, David Pierce, Paul Popa, Yanxiang Li and Paul Foley. Dow Isocyanate Free Polyurethane Coatings – Fundamental Chemistry and Performance Attributes, European Coatings Conference, April 21, 2015.
Zeno W. Wicks Jr., Frank N. Jones, Socrates Peter Pappas, Douglas A. Wicks. (2007). Organic Coatings: Science and Technology, Third Edition.
Wiley, Jones e.al. (2017) Organic Coatings, Science and Technology, Third Edition.
