ORIGINALLY POSTED IN THE EUROPEAN COATINGS JOURNEY 07/08/2019
A new generation corrosion control coating technology with high crosslink density. By Atman Fozdar, Ronald Lewar- chik, Raviteja Kommineni, Chemical Dynamics LLC, USA.
An innovative technology that offers improved performance, saves material and labour costs and eliminates the need for an epoxy primer coat. A single component polymeric penetrant reacts with the corroded base metal to form a long- lasting bond and increase the structure’s useful service life. This coating technology has far-reaching potential, for example in off-shore applications, chemical processing and automotive re- finishes.
Mild steel is one of the most used alloys for different kinds of applications be- cause of its low cost, abundant supply and easy fabrication. But corrosion of steel is one of the major issues faced by transport (e.g. automobiles, aircraft, ships) and infrastructure (e.g. pipelines, buildings, bridges, oil rigs, refineries) industry which directly affects its structural integrity, resulting in issues related to safety and maintenance of steel structures. According to the research published by NACE International [2], corrosion is responsible for losses over $ 2.5 trillion every year. There are different methods to counter corrosion such as, using corrosion inhibitive lining, electroplating, organic polymeric coating and chemical vapor deposition. Ap- plying protective organic coatings to metallic substrate, especially aluminium and steel, is an effective way to protect those substrates against severe corrosive environments. Organic coatings can minimise corrosion of metallic substrates by three main mechanisms: barrier, sacrificial and inhibition.
We often see early signs of corrosion on a steel structure for a variety of reasons. It may be caused by poor surface preparation or application of protective coatings or possibly environmental factors such as acid rain, high humidity, temperature variations, condensation of moisture, chemical fumes, and dissolved gases in case of structures submerged in water or soil. Among the factors listed above, improper surface preparation is one of the most important factors that contributes to the corrosion of steel structures and can lead to loss of structural integrity and structure before the end of its useful service life. If there is a way to protect the structures after observing initial signs of corrosion, without going through labour-in- tensive tasks such as coating removal, clean- ing, pre-treatment and recoating application, then this can significantly increase its service life, more efficiently and economically.
Results At A Glance
- We have developed a single component polymeric penetrant that can be applied with or without surface preparation over clean or lightly corroded steel/aluminium.
- The coating contains nanosized reactive materials which first penetrate the rust and then migrate to the non- corroded metal surface, polymerising to form a highly crosslinked and protective network.
- Results over cleaned pre-treated steel surfaces can exceed 10,000-hour salt spray with no blisters or scribe creep when top coated.
- The new innovative technology offers improved performance, eliminates the need for an epoxy primer coat, and saves labour and material costs.
Experimental
One unique aspect of low molecular weight oligomers used in RA Exp1, is a prevalence of three types of reactive unsaturation on the resin backbone and low molecular weight reactive diluents. The three types of double bonds offer a synergistic curing mechanism that results in ancillary curing properties and high crosslink density that inhibits the penetration of soluble salts and moisture. Corrosion resistance is further improved when this resin blend is coupled with corrosion inhibitor pigments such as organically modi- fied zinc aluminium molybdenum orthophosphate hydrate and zinc-5-nitroisophthalate and unique conductive particles. Graphical representation of how RA Exp1 penetrates rust is shown in Figure 1. After penetrating the surface of the substrate, low molecular weight unsaturated monomers and oligomers, chemically bond/crosslink with other reactive sites, forming a highly crosslinked network which is impermeable to moisture and other soluble salts responsible for aggravating corrosion.
Hydrophobic and superhydrophobic variations of RA Exp1 were produced by adding superhydrophobic nano-textured silica [3]. This additive is naturally superhydrophobic having both hydrophilic/phobic sites and produces a volumetric hydrophobic coating. Hence, even if the surface of the cured coat- ing is abraded due to normal wear and tear experienced in the field, the underlying layers will still repel moisture. We formulated a separate design of experiments for RA Exp1 (with and without the additive) and 2-component polyurethane topcoat (with and without the additive).
Protection Demonstrated In Salt Spray Testing
Variations of RA Exp1 with and without the additive were applied on zinc nickel treated cold rolled steel substrate, which was later top coated with a 2k polyurethane coating with and without the additive at 125 μm dry film thickness (DFT) each. A salt spray test was performed in a salt spray cabinet in accordance with the ASTM B117 standard, after which all the panels were cured at ambient temperature for 7 days. Coated panels with an artificial defect (scratch with a dimension of 106 mm x 2 mm, created using a 1 mm scribe tool) were used to accelerate the corrosion process. All coated panels were placed in a test chamber at an angle of 45 ° and ex- posed to the 5.0 wt.% NaCl solution at 40 °C. The condensate collection rate and relative humidity were at least 1.0 to 2.0 ml/h per 80 cm2 (horizontal collection area) and 95 %, respectively. The protective performance of the coating was further investigated with the emphasis on size and distribution of corroded or damaged area on the coated sample surfaces after 10,000 hours of salt spray exposure.
Figure 2 shows 10,000-hour salt spray expo- sure, three of the four systems with RA Exp 1 as the primer and a 2K polyurethane topcoat show no scribe or face blister and/or corrosion. The top four photos show different systems after 10,000 hours of salt spray expo- sure and the bottom four photos show the extent of corrosion underneath the coating (of the same systems) after removing bottom half of coating using paint stripper.
Low Impedance Due To Conductive Nanoparticles
The barrier protection properties of RA Exp1 was investigated by performing EIS on Zinc phosphate pre-treated cold rolled steel, the results of which were compared with those of commercially available coatings based on conventional 2-component epoxy and moisture-cured urethane system. A three- electrode paint test cell (reference electrode: saturated Calomel electrode (SCE), counter electrode: working electrode: steel samples in 14.6 cm2 area) was used to perform the EIS measurements [1]. Impedance quantifications were made at open circuit potential (OCP) which were maintained potentiostatically in the frequency range of 0.1 to 100 KHz and at amplitude sinusoidal voltage of ± 60 mV. The four samples (RA Exp1, 2k epoxy and two moisture-cured urethane samples) were immersed in 40 mL NaCl solution (3.5 wt.%) and EIS measurements were per- formed over a period of 40 days.
Initial Bode and Nyquist plots (Figure 3a & 3b respectively) indicate that all coating variations show a capacitive behaviour with high impedance values. RA Exp1 was found to have relatively lower impedance values compared with other control samples, which could be attributed to the conductive/anti-static nature of the coating due to the addition of conductive nanoparticles and additives to enhance corrosion resistance.

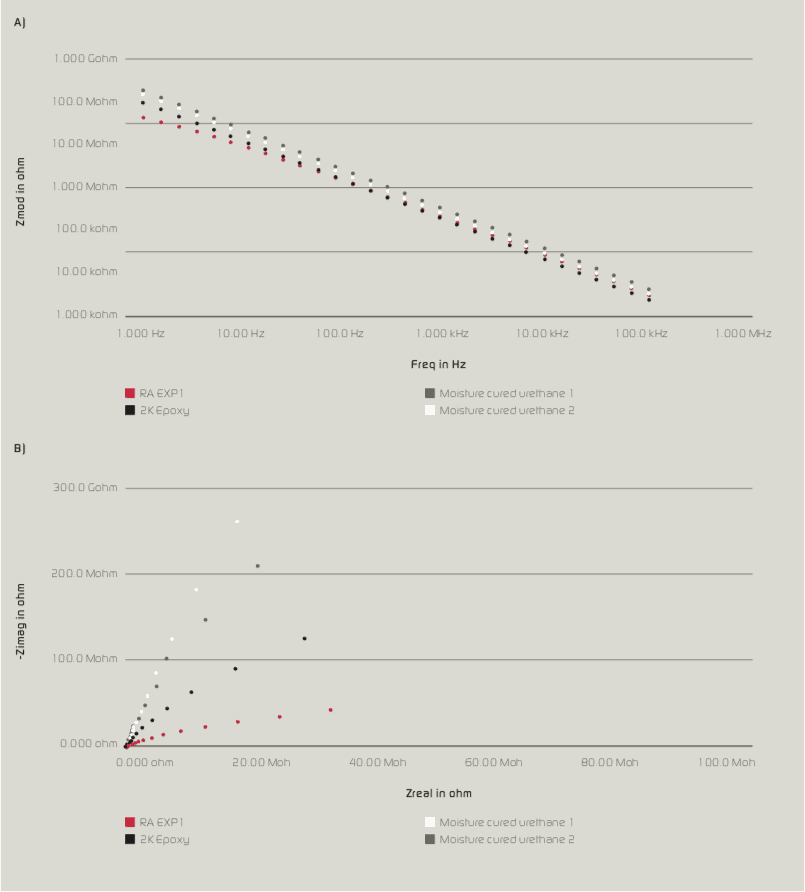
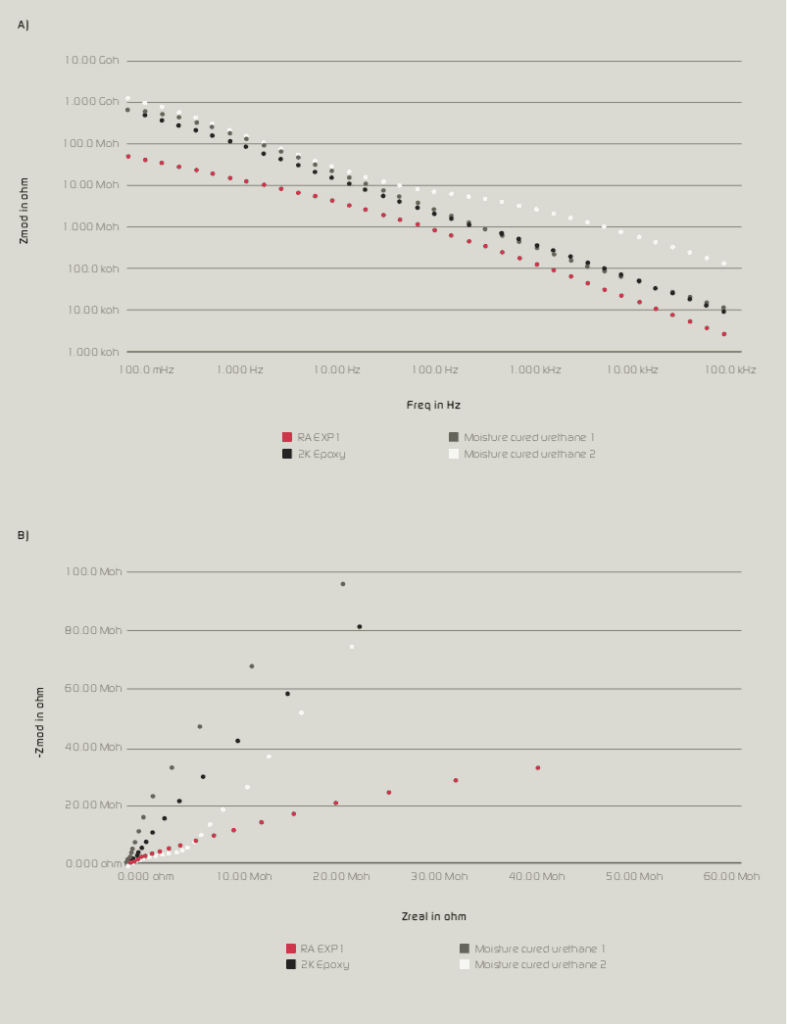
Figure 4b: Nyquist plot of RA Exp1, 2K Epoxy, Moisture cured urethane 1 & 2, after 50 days (1,000 hours) of exposure.
Greater Resistance To Electrolyte Diffusion
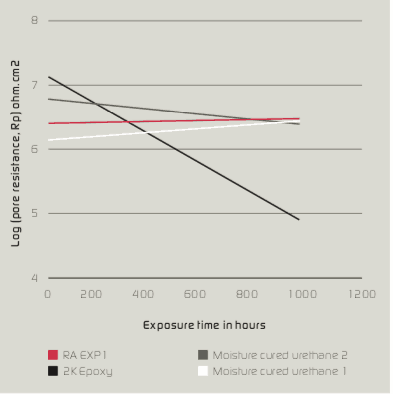
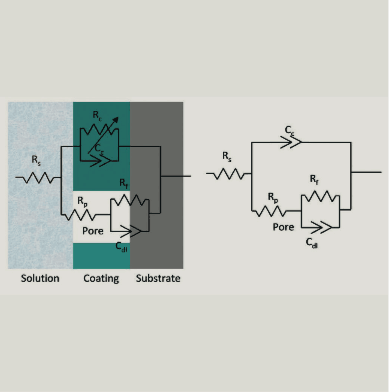
Figure 6 shows a simplified equivalent circuit for a metal substrate protected by a semi-permeable coating layer, ignoring the coating resistance of negligible magnitude. The values of circuit elements in equivalent circuit networks can be used to directly characterise coating performance. Pore resistance (Rp) values extracted by fitting equivalent circuit model as a function of exposure time can be used to compare the performance and rank various coating systems. Figure 5 shows a plotted graph containing the logarithm of pore resistance (RP) vs. exposure time (hours), which indicates that Rp of 2K epoxy decreases with time whereas, RA Exp1, moisture-cured ure- thane 1 and urethane 2 are nearly constant for 1,000 hours of exposure to a 3.5 % NaCl solution.
After 1,000 hours of immersion time, impedance values of moisture-cured urethane samples 1 & 2 decreased significantly while RA Exp1 and 2K Epoxy were able to maintain their impedance values without showing a significant decrease. As shown in Figure 4a & 4b, the behaviour of moisture-cured urethane 2 changed from 1 to 2 constant. This could be due to the diffusion of electrolyte to coating and substrate interface; hence, a double layer could be formed below coating layer. For other samples including RA Exp1, no such behaviour was observed which suggests the coating layer was more resistant to the diffusion of electrolyte and soluble salts.
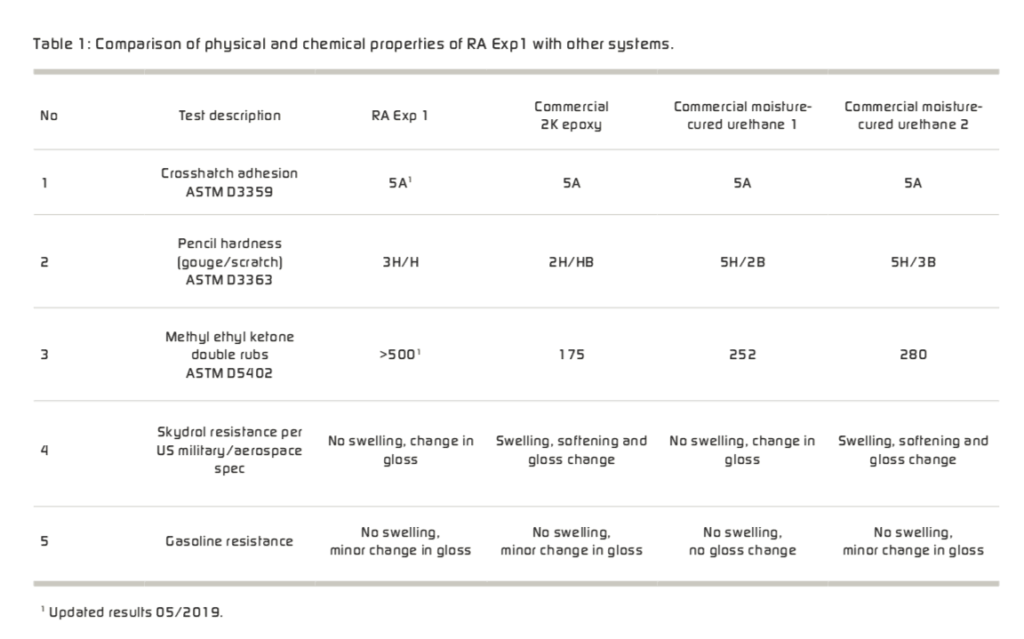
RA Exp1, 2K Epoxy and various moisture-cured urethane systems were spray applied on clean zinc phosphate pre-treated cold rolled steel and sanded cold rolled steel panel at 125 μm dry film thickness (DFT) and were allowed to cure at ambient temperature for a period of 7 days before characterising the physical and mechanical properties. Table 1 provides a com- parison of the physical and chemical properties of the new technology with other systems.
Potential Use In Extreme Conditions
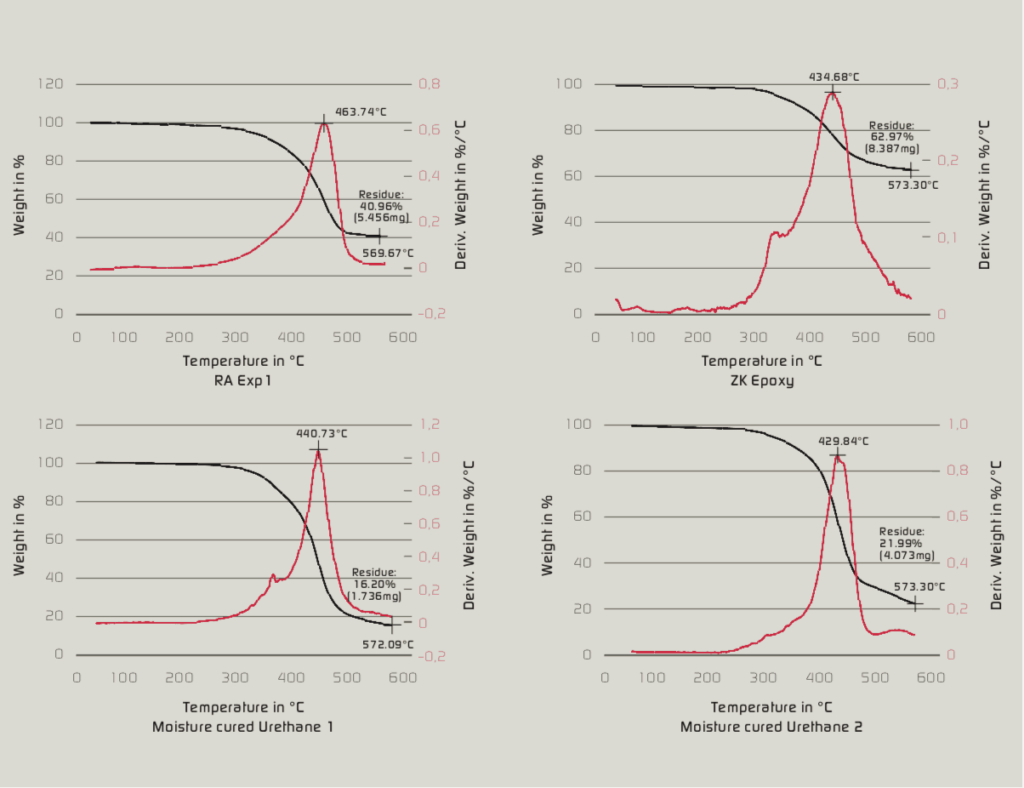
Thermogravimetric analysis (TGA) was performed on RA Exp1, 2K Epoxy and moisture-cured urethane 1 & 2. The results indicate that RA Exp1 has comparatively higher decomposition temperature of 463.74 °C, whereas the decomposition temperature of other coatings ranges from 430-440 °C (Figure 7). This study confirms that the RA Exp1 can potentially be used in an environment where coatings are exposed to extreme conditions such as high heat i.e. boilers, chemical processing equipment, pressurized vessels etc.
High-Performance Two-Coat Corrosion Protection
The novel technology represents a dramatic enhancement in the corrosion resistance of metal substrates such as: pre- treated aluminium, zinc-nickel treated cold rolled steel, lightly rusted steel and zinc phosphate treated cold rolled steel coated with RA Exp1. Results demonstrate better face blister resistance, scribe creep resistance and overall better corrosion resistance per ASTM B117 than all other systems tested in this scope of work. The higher decomposition temperature per TGA analysis indicates a potential use of RA Exp1 for high temperature applications. The reaction kinetics of different vinyl polymerisation reactions and oxidative cure of RA Exp1 are not fully defined and still remains a subject of investigation.
The potential applications for this technology include: high-performance protective coatings for maintenance and repair application, automotive refinishing, industrial application, product finishing, offshore application such as oil rigs and refineries, the ACE industry, as well as boilers, chemical processing equipment and pressurised vessels.
In conclusion, this new generation of innovative protective coatings and superhydrophobic protective coatings provide the industry unsurpassed corrosion protection in a two- coat system.
3 questions to Atman Fozdar
What temperature do you recommend for curing to achieve an optimal effect? Coating can be cured at ambient temperature similar to how most coatings are cured for maintenance and repair applications in the field but cure can also be accelerated by thermal bake. For ambient condi- tions, full properties are achieved after 7 days.
Did you test the laboratory results under reality conditions? Subject coating has been applied on multiple substrates such as cold rolled steel, zinc phosphated cold rolled steel, hot rolled steel, 2024 & 7075 Aluminum pretreated with hexavalent chrome sealer, Cadmium treated panels (used in aerospace) along with zinc-nickel treated substrates (used in aerospace and automotive). Acceler- ated properties such as UV-A exposure, ASTM B117 salt spray, Cleveland condensing humidity test along with real life exposure in some of the warmer climate regions near coastal areas are currently being tested.
Are the high temperature loaded films you mentioned still corrosion resistant? Coated objects exposed to temperature in excess of 350–400 °C but less than 450 °C along with saturated steam exposure are performing well after few weeks of salt spray exposure (ongoing test). However, this test was performed in a controlled lab condition. Field evaluation is still a subject of investigation.
[1] MertenB.,CoatingevaluationbyElectro- chemical Impedance Spectroscopy (EIS) Report “ST-2016-7673-1” 2015.
[2] NACEInternational-https://inspectioneering. com/news/2016-03-08/5202/nace-study- estimates-global-cost-of-corrosion-at-25-trillion- ann. 2016
[3] Simpson J. et al. 2015 Rep. Prog. Phys. 78 086501.
Featured photo: Source: Nikolay Zaburdaev – stock.adobe.com

