Ancient civilizations including those in Egypt, China and India have utilized metals or metal compounds utilizing copper, silver and zinc to combat illnesses caused by microbes, while the ancient Greeks and Egyptians used specific molds and plant extracts to treat infections. Since the arrival of SARS, and more recently COVID 19, there is an increasing awareness and use of antimicrobial materials including antimicrobial coatings to combat the spread of disease-causing microbes. The estimated market value of antimicrobial coatings was over $3.2 Billion USD in 2019 with an estimated adjusted annual growth rate of 10.4% through 2026.
Antimicrobial (AM) agents in the form of paint additives act to either kill microorganisms or to stop their growth. Antimicrobial additives in paints can serve as a paint preservative or as an antimicrobial agent in the cured film. Depending on the choice of antimicrobial additives these materials can function to kill or combat the growth of bacteria, virus, fungus and algae on the coating surface. Control of microbes can be achieved through the use of antimicrobial technologies that keep microorganisms from multiplying or growing, providing hygienic surfaces in hospitals and the food industry and to preserve the integrity of paint films.
This article will focus on antimicrobial additives and approaches to provide antimicrobial functionality in cured films. Applications where AM agents are used in coatings to kill or prevent the growth of the following microbes including:
- fungi
- bacteria
- algae
- virus
Most biocides used in paints are migratory as they function by releasing the active ingredient to the surface of the coating when exposed to moisture. Longevity of the AM modified paint film depends on the rate of release of the biocide as the concentration of the active ingredient decreases with time.
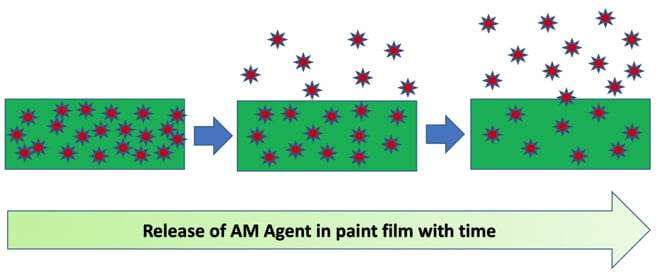
The effectiveness of an AM additive in a cured paint is not only dependent upon concentration, resin system, gloss, PVC, coating surface structure and the environment to which it is exposed.
The use of metals such as silver, copper (and many copper alloys ) and zinc in various forms in paints can be an effective antimicrobial additive. There are several mechanisms by which silver acts as an antimicrobial. One such example is that silver ions react with the thiol group in enzymes leading to cell death. The mechanisms through which copper acts to destroys cells includes the generation of hydrogen peroxide in the cells, excess copper can also bind with proteins resulting in the breakdown of the protein into nonfunctional sections. Zinc pyrithione/2-propynyl butylcarbamate acts both a preservative and as a fungicide. The EPA oversees the regulation of antimicrobial agents and materials and determined that copper alloys kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly. Copper and copper alloys are unique classes of solid materials as no other solid touch surfaces have permission in the U.S. to make human health claims. Accordingly, the EPA has granted antimicrobial registration status to 355 different copper alloy compositions.
Metal nanoparticles including PVP and polysaccharide coated silver nanoparticles, MES-coated silver and gold have also demonstrated promise as antiviral agents. Copper nanoparticles have demonstrated antimicrobiological activity with Ecoli, fungus and bacteria.
The use of certain Quaternary Ammonium Silane compounds also provide antimicrobial properties when bonded to a solid surface. Some examples include dimethyloctadecyl (3-trimethoxysilyl propyl) ammonium chloride, alkyldimethylbenzylammonium chloride and didecyldimethylammonium chloride.
More recent literature reveals the impact that surface structure has on antimicrobial properties as a needle like surface structure formed by the bonding of 3-(trihydroxysilyl) propyldimethyloctadecyl ammonium chloride to the surface to destroy microbes by rupturing their outer membrane as they come in contact with surface spikes.
Chemical Vapor Deposited titanium dioxide has photocatalytic activity when exposed to UV light. Its self-cleaning properties are due to its strong oxidizing power that results in anti-bacterial, anti-viral and anti-fungal activity.
Superhydrophobic surfaces are those with a contact angle normally in the range of 150 degrees or greater. The surface structure is characterized by a needlelike micro-structure coupled with components that provide low surface tension. Such surface structures also have efficacy in reducing the ability of microbes to adhere to the surface thus imparting antimicrobial activity.
For additional information concerning the selection of materials to enhance hydrophobicity, please navigate to www.ulprospector.com (EU).
Resources:
- Organic Coatings, Science and Technology, Frank N. Jones et.al., Wiley & Sons, 2017
- Prospector
- PCI Magazine
- C & EN News
- Science and Technology of Advanced Materials
- Wikipedia
- Global Market Insights
