Original article date: Nov. 29, 2019
Updated Dec. 12, 2022
Paint films for nearly all aesthetic and functional applications above all else must provide adhesion to the desired substrate. Secondly for long term durability, the coating must continue to provide tenacious adhesion during the service life of the coating. Accordingly, one must take into account multiple considerations when formulating a coating that provides acceptable adhesion for the intended application. Critical considerations and how they impact adhesion include:
- Surface wetting
- Mechanical effects and internal stress
- Maintaining film Integrity and intercoat adhesion
- Surface chemistry and bond strength
- Pigmentation
- Evaluation of adhesion both initially and after accelerated testing
1. Surface wetting
The relationship between surface wetting and adhesion is the first factor to be considered in designing a coating to optimize adhesion. If a coating in a liquid state does not spread spontaneously over the substrate surface, then there is limited opportunity to form mechanical and chemical bonds with the substrate surface.
A liquid will spread spontaneously on the surface of a material if the surface tension (force/unit length or dyne/cm) of the liquid is lower than the surface free energy of the solid to be coated. For example, the image below provides a visualization of various degrees of wetting properties for a drop of liquid applied onto the surface to be wet.
Figure 1 – Images of Various Degrees of Substrate Wetting
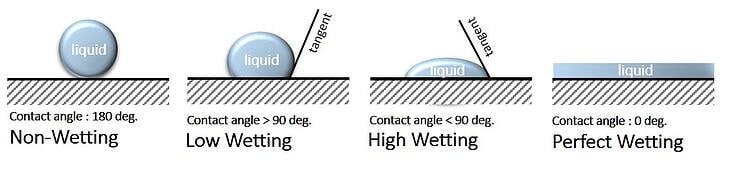
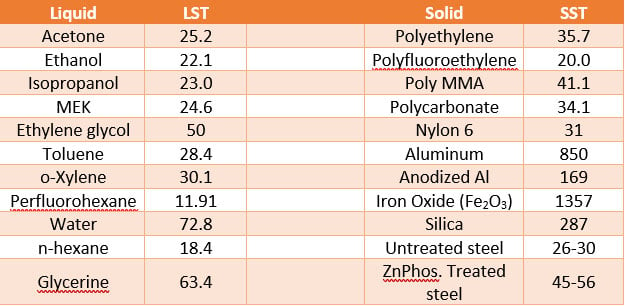
Accordingly, in Table 1, when the Liquid Surface Tension (LST) is lower than that of Solid Surface Tension (SST), then wetting of the solid will occur. The greater this difference, the greater the opportunity the liquid has to wet and spread on the surface of the solid. Accordingly, to improve wetting as the initial step to gaining adhesion, either the LST can be decreased and/or the SST can be increased. Waterborne paints and powder coatings have a more difficult time spreading on surfaces due to the relatively high surface tension of water or that of a powder wetting in comparison to most paints containing a higher level of organic solvents to provide wetting.
Accordingly, to improve wetting of powder coatings and waterborne coatings, organic cosolvents (for waterborne) and/or appropriate wetting agents (waterborne and powder) are normally employed. In summary, when LST < SST, wetting occurs.
Table 1 – Liquid Surface Tension (LST) and Solid Critical Surface tension (SST) (dynes/cm) @ 20° C
2. Mechanical adhesion and internal stress
The profile of the substrate the coating is to be applied to also can affect adhesion. Smoother surfaces are more difficult for coating adhesion as the surface area is lower and provides less area for the coating to interlock with the substrate. However, if a coating is extremely rough, it can be difficult for a liquid coating to wet and penetrate surface crevices. This is illustrated in the diagrams listed below in Figure 2.
Figure 2 Surface interactions between a coating and substrate
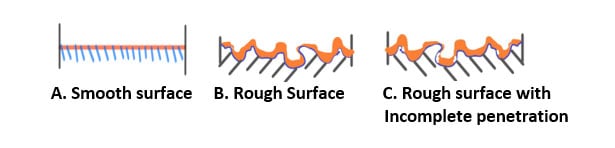
The microscopic surface profile in sketch B will provide better adhesion than that in sketch A as the coating provides greater opportunity to interlock with the substrate. Surface C has pockets and pores that are not easily penetrated by the coating, resulting in air pockets that can trap moisture and soluble ions resulting in blisters and corrosion (if substrate is an oxidizable metal) and thus poor long-term adhesion and eventual film failure.
In summary, from a mechanical adhesion standpoint, liquid coatings with low surface tension and low viscosity help promote better wetting and microscopic penetration (capillary action). Adhesion can also be adversely affected by stresses that occur as a result of shrinkage as a coating dries or cures. Environmental effects over time such as exposure to moisture, light, heat, pollutants and thermocycling also play an eventual role to degrade adhesion.
3. Maintaining film integrity and intercoat adhesion
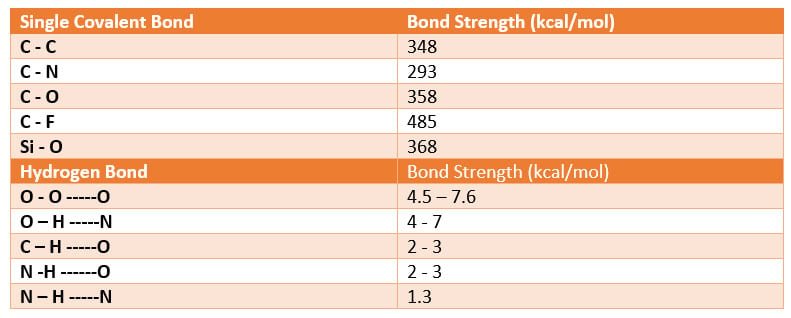
To maintain film integrity and intercoat adhesion in multicoat systems such as topcoat to primer or clearcoat to color coat to primer, mechanisms such as interfacial mixing during application and/or cure helps promote intercoat adhesion, a second mechanism which provides a further enhancement of intercoat adhesion is the reaction of residual reactive functional groups on one layer of a multicoat system to react and form covalent bonds with that of functional groups of another coating layer. Other means to improve substrate adhesion and or intercoat adhesion include the addition of adhesion promoters (see references listed below) and/or hydrogen bonding to adjoining surfaces. Bond strengths of covalent bonds are orders of magnitude stronger than that of hydrogen bonds and thus preferred to maintain long term film integrity from a longevity standpoint.
4. Surface chemistry and substrate bond strength
In addition to surface tension and surface profile of the substrate, available substrate functional groups may provide sites for covalent and hydrogen bonding to the coating components to further enhance the adhesive bond strength to the substrate.
Table 2 – Adhesive bonding forces
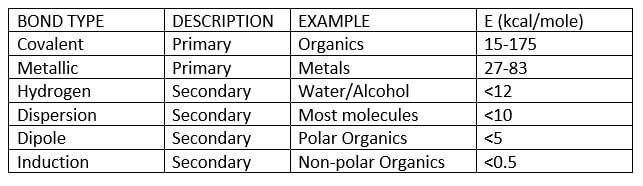
As Table 2 illustrates, the highest bond strength to the surface is provided by covalent bonds, such as those provided for example the reaction of a dual functional trialkoxy silane coupling agent between the coating and the metal surface.
Most metal surfaces are supplied with a thin layer of oil to slow the rate of oxidation. The oil also lowers the surface energy and thus is more difficult to wet. For this reason, metal surfaces -for example steel, zinc coated steel and aluminum- are normally cleaned prior to painting to remove oils and then pretreated to form, for example, a zinc phosphate or iron phosphate treated surface. The phosphate groups serve to enhance adhesion of the coating through hydrogen bonding of the metal surface to reactive sites on the polymer.
Figure 3 Example of Hydrogen bonding to a metal surface pretreated with Zn.Phosphate
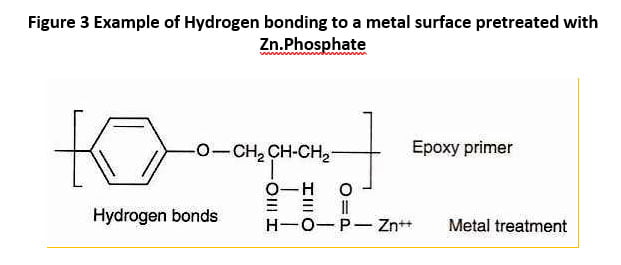
Reactive groups on the polymer back bone or through the addition of a di or multifunctional adhesion promoter containing epoxy, amino or silane functional coupling groups can further react with a suitable pretreated metal surface to form covalent bonds that provide added adhesive strength between the metal and the coating.
For glass or silica-rich surfaces, coupling agents such as amino silanes can also serve to enhance adhesion by reacting with a resin backbone containing an epoxy group with the alkoxy functional silane portion of the coupling agent bonding to the silica surface to form a siloxane.
Plastics are more difficult to wet as they have a lower surface free energy that may be further lowered by the presence of mold release agents. Adhesion to polyolefins can be improved by increasing their surface free energy through UV irradiation, once a photosensitizer is applied, or flame treatment that generates hydroxyl, carboxyl and ketone groups.
These functional groups on the plastic surface provide higher surface energy to improve wetting as well as hydrogen bonding sites for polymer functional groups on the coating. Other ways to improve adhesion to thermoplastics is to include an appropriate solvent in the paint to solubilize the plastic surface and enable intermixing of the coating at the plastic-coating interface.
5. Pigmentation
The level and type of pigment used in a primer not only affects coating substrate adhesion, but also how long it will adhere to the surface. Most primers are formulated at or slightly below Critical Pigment Volume Concentration (CPVC) to maximize topcoat adhesion (rougher primer surface and higher free energy) as well as many other coating properties (Figure 4).
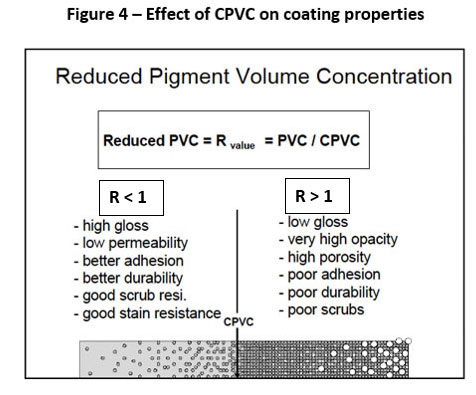
The use of more polar pigments may provide ease of wetting during the pigment dispersion process, but may degrade long-term adhesion as they are more susceptible to moisture migration and disbondment at the coating-substrate interface. Plate-like pigments and pigments that have very low or no water-soluble components also enhance longevity.
6. Evaluation of adhesion
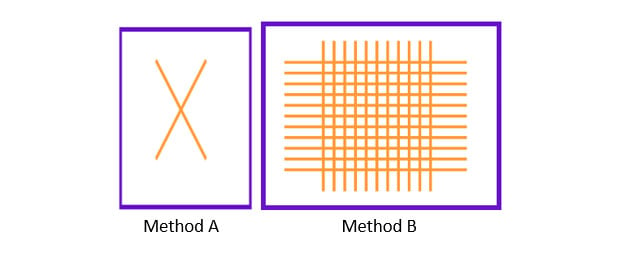
There are multiple ways to determine and quantify the adhesion of organic coatings to a substrate. Two of the most common means of determining adhesion include ASTM D3359 (Cross Hatch Tape Adhesion) and ASTM D4541 (Pull-Off Adhesion). ASTM D3359 describes two methods to determine cross hatch tape adhesion: method A is a simple X, where method B is a lattice pattern. Method A is used in the field and for films > 5mils, whereas Method B is used for lab determinations.
ASTM D3359 Ratings are by area of the cross hatch removed by specialized adhesion tape and include:
5B (no area removed) > 4B (less than 5%) > 3B (5 – 15%) > 2B (15 – 35%),1B (35 – 65%) and 0B (greater than 65%)
ASTM D4541 (Pull-Off Adhesion) utilizes a device to measure the Pull Off Strength of a dolly glued to the surface of the coating. The device determines the force required to disbond the coating in pounds per square inch. This not only quantifies the amount of force required to pull off the coating, but also the type of failure (cohesive or adhesive), how and at which layer the coating fails (topcoat to primer, primer to substrate etc.).

Sources:
- Metal Surface Treatment – The Key to Successful Performance, Ron Lewarchik, 4 November 2016
- Achieving Superior Coatings Adhesion, Jochum Beetsma, 13 June 2014
- Reactive Silanes for Enhancement of Coating Performance, Ron Lewarchik, 6 March 2015
- Adhesion Promoters 101, Marc Hirsch, 19 May 2016
- Pharmacy 180.com
- Organic Coatings, Science and Technology, Frank N. Jones et.al., Wiley & Sons, 2017
- Prospector Knowledge Center
- www.surface-tension.de
- Science Direct
- Science & Technology AJ Kinloch, Chapman & Hall
- CSCScientific.com
- ASTM Standards
- www.defelsko.com
