A common ambient cure two-component paint chemistry involves the reaction of an epoxy resin with that of an amine functional resin (hardener). Due to their tenacious adhesion and moisture resistance, crosslinked epoxy resins are used on a variety of surfaces including metals and concrete, epoxy two-component compositions are used in a variety of applications including primers for exterior and interior applications.
Reactivity of epoxy groups with amine hardeners
As Table II illustrates, epoxy groups react with primary amines at ambient temperatures to form secondary amines that can in turn react to form tertiary amines. In terms of reaction rate of various epoxy and amines, Table I lists a few general structural-reactivity relationships of epoxy groups with amines hardeners.
Table I – General comparison of the reactivity rate of functional groups in epoxy and amine hardeners
| Amine reactivity | Primary > Secondary > Tertiary |
| Amine reactivity | Reaction rate increases with increasing base strength |
| Amine reactivity | Decreases with an increase in steric hindrance |
| Amine reactivity | Aliphatic > aromatic or cycloaliphatic |
| Epoxy reactivity | Aromatic (for example a bisphenol A based epoxy) > Aliphatic (ie. hydrogenated version of Bisphenol A based epoxy) |
| Epoxy reactivity | Terminal epoxy groups > internal epoxy groups |
Table II – Example of reactions of epoxy with amine
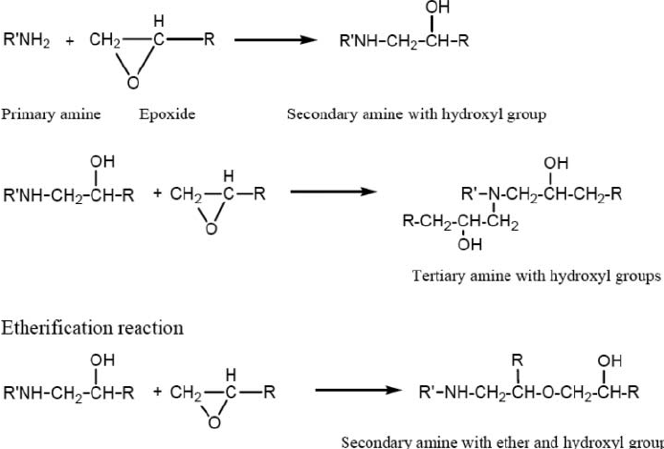
With the correct catalyst, aliphatic epoxy resins can react with carboxyl functionality even at room temperature. Cycloaliphatic epoxy-based systems (ie. Using hydrogenated BPA as a building block) also provide improved light stability for exterior applications.
When formulating a stoichiometric reaction, it is desirable to discuss reactants in terms of equivalents.
Calculating mix ratios of epoxy – polyamine
For example, to calculate the stoichiometric parts by weight of hardener per 100 parts per hundred weight of epoxy resin:
phr = Amine Hardener Eq. Wt. X 100 / Epoxy Eq. Wt. of resin
For example, if the epoxy resin Eq. Wt. = 400 and the amine hardener Eq. Wt. is 100
The phr = 10000/400 = 25.0
Accordingly, 25.0 parts of amine curing agent are needed to cure 100 parts of epoxy resin for a 1:1 stoichiometric ratio of amine hardener: epoxy resin.
Epoxy resins
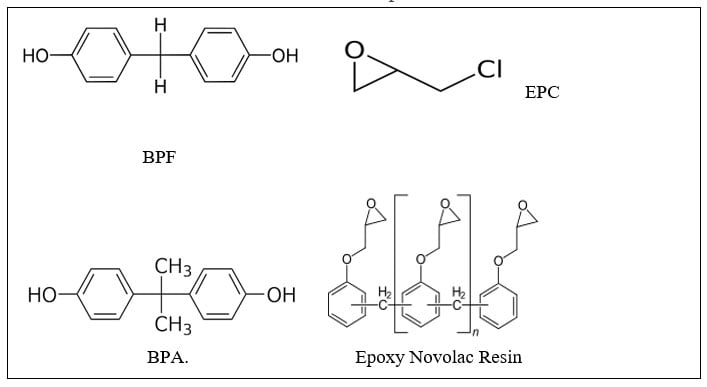
Per the diagram below, most epoxy resins are made by reacting Bisphenol A (BPA) with an excess of epichlorohydrin so the end groups are glycidyl ethers. The molecular weight and the epoxy equivalent weight are controlled by the ratio of epichlorohydrin EPC:BPA. Bisphenol F (BPF) based epoxy resins are more flexible than that of BPA-based epoxies. Hydrogenated BPA based epoxy resins provide improved exterior weathering as the aromatic groups that absorb UV are absent. Other epoxy resin types include epoxy-novolac (EN) and epoxy-phenolic (EP). Epoxy-novolac (EN) resins provide a higher crosslink density as they have a higher functionality (epoxy functional side chains) and thus provide a higher crosslink density and better chemical resistance. Epoxy-phenolics are also known for their chemical resistance coupled with excellent corrosion resistance.
Other hardeners
In addition to amine functional hardeners for use in curing of epoxy-functional resins, polyamides, amidoamines, phenalkamines and mercaptan functional curing agents provide improved low temperature cure rates. For example, polymercaptans cure with epoxies at 0 C to – 20 C. Solvent selection is another important factor in formulating epoxy-amine two component systems. Suitable hydrogen acceptor solvents such as t-butyl acetate can prolong pot life. Most ketones and esters (except for TBA) should be avoided since they form ketimines especially with primary amines at room temperature and this results in a reduction in the amount of active amine. Alcohols also slowly react with epoxy groups at room temperature. If mono-alcohols are used there is little change in viscosity. However, over time, this decreases the number of epoxy functional groups present and results in a reduction of the crosslink density of Part A containing epoxy resin and a primary alcohol solvent.
Accelerators
Tertiary amines act like cure accelerators, along with water, some alcohols, and some weak acids such as phenols. For example, 2,4,6-[Tris(dimethylaminomethyl)] phenol has both phenolic and tertiary amine groups and is also an effective catalyst. Weak acids promote the ring opening reaction of the epoxy.
The UL Prospector search engine provides a number of amine hardeners and epoxy resins for formulating two-component and other types of epoxy coatings.
Further Reading and Sources:
- Prospector Knowledge Center:
- Organic Coatings, Science and Technology, Third and Fourth editions
- Wikipedia
