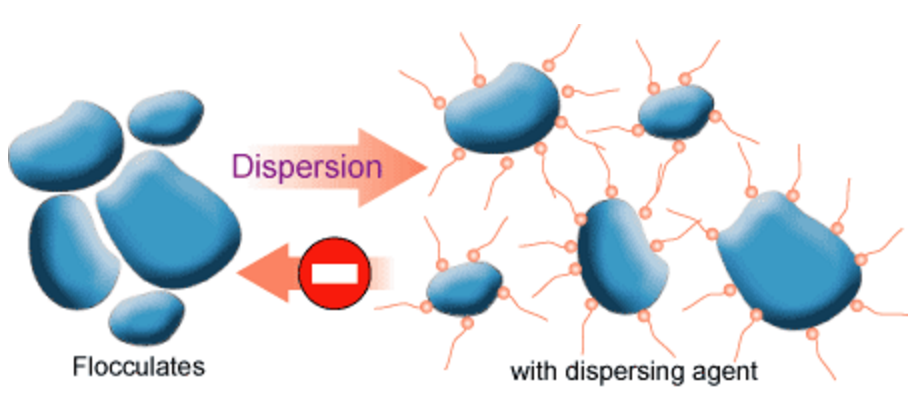
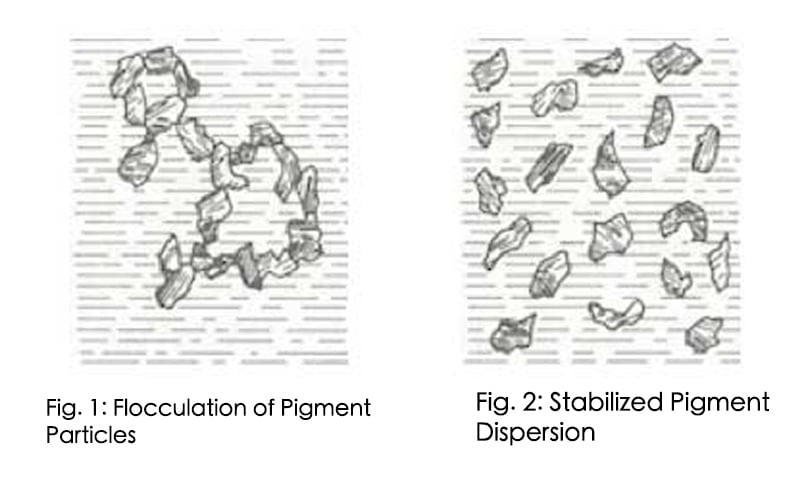
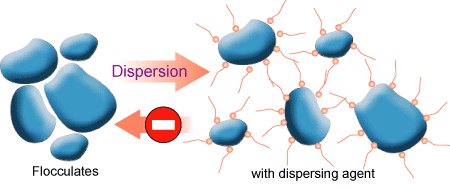
Introduction -The first steps in the pigment dispersion process are wetting and separation of the pigment. However, if the pigment dispersion is not properly stabilized, flocculation1 (fig. 1, 2) will result. Flocculation is a result of pigment particles being attracted to each other to form loose aggregates that can be redispersed under mild shear.
When pigment particles are strongly attracted to one other a cementing or agglomeration of the particles can occur. Agglomerates (chemically bound pigment aggregates that are encapsulated by resin or wetting agent) cannot be readily redispersed. Flocculation can be reversed by the application of low shear to the paint. Flocculation can have an adverse effect on color development, gloss and hiding.
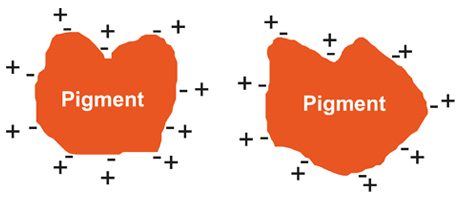
The two main mechanisms to obtain pigment stabilization are steric and charge. In charge repulsion, particle surfaces with like charges repel each other (more applicable to waterborne systems, Fig. 4) whereas steric stabilization is a more common mechanism in solvent born paints (Fig. 5). Properly stabilized pigment dispersions prevent flocculation and agglomeration.
Pigment dispersion in aqueous media uses the same principles as inorganic solvent media: that is, proper wetting, pigment dispersion and stabilization. However, the surface tension of water and high polarity makes it more problematic in wetting low polarity pigments. In many cases, water interacts aggressively with the surface of the pigment, destabilizing the dispersant on the pigment surface. Ensure that the pigment dispersion is uniform and stabilized (elimination of pigment flocculation of one pigment with the exclusion of other pigments). Thirdly, the use of suitable wetting agents/surfactants help to ameliorate differences in polarity and surface tension between pigments that contribute to pigment destabilization.
Inorganic pigments such as iron oxides, titanium dioxide, calcium carbonate, zinc oxide, and silicon dioxide, calcium carbonate and barium sulfate and many other filler pigments have a very polar surface. However, water alone normally does not adequately wet the pigment surface. Accordingly, they require a surfactant to wet and stabilize the dispersion.
Also, many pigment manufacturers supply surface-treated pigments to help pigment stabilization. Many manufacturers modify the surface of organic pigment to increase polarity by adding a layer of inorganic oxide to improve pigment wetting.
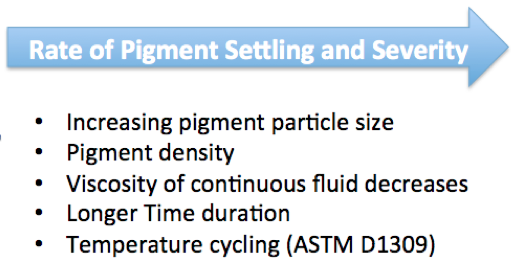
No discussion on pigment stabilization is complete without considering the effect of pigment settling with time. These factors all influence the degree of pigment settling and resistance to hard settling:
- Quality of the pigment dispersion
- pigment particle size
- oil absorption
- shape
- distribution
- pigment density
- paint viscosity
A more complete discussion of the impact of each of these parameters on pigment hard settling and stability would require several articles to adequately describe. However, Figure 6 provides a simplified relationship of pigment and paint parameters to pigment settling.
Finally, the use of an appropriate thixotrope helps to build sufficient viscosity and a network structure that discourages pigment hard settling. A suitable thixotrope can improve resistance to hard settling by a few different mechanisms.
- Improves resistance to hard settling by increasing low shear viscosity
- Forms an association with the pigment to decrease the effective density of the settled pigment layer.
However, one must be sure that there is acceptable compatibility between the thixotropic and dispersant. Thixotropes commonly used to promote soft settling include clays treated with quaternary ammonium compounds to provide higher organophilicity for solvent born coatings. Attapulgite clays are used in both waterborne and solvent born coatings, as the needle like clay particles associate to increase viscosity that easily breaks down under shear. Other polymeric thickeners can be effective by increasing viscosity and by promoting readily redispersible soft settling, such as:
- Fine particle silicas
- castor oil derivatives
- basic calcium sulfonate
- colloidal aluminum silicate
To read the rest of the article please click here to head over to UL Prospector.
__________
Ron Lewarchik, Author of article & President of Chemical Dynamics
As a contributing writer, Ron pens articles on topics relevant to formulators in the coatings industry. He also serves as a consultant for the Prospector materials search engine, advising on issues related to optimization and organization materials within the database.