Acrylic Resin Fundamentals, UL Prospector, Ronald Lewarchik, 4/2016:
Coatings utilizing acrylic resins are the leading polymer technology in the coatings industry. Historically alkyd finishes have held the leading position in coatings for decades. Acrylics are utilized in architectural coatings, product finishes for original equipment manufacture including automotive (OEM) and refinish, as well as special-purpose coatings.
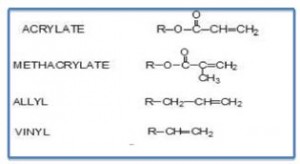
Acrylic resins are primarily based on acrylate and methacrylate monomers and provide good weather resistance, resistance to hydrolysis, gloss and color retention in exterior applications. Due to their versatility and performance, acrylic coatings account for over 25% of all coatings and global sales approaching $25 billion. Acrylic resins can be thermoplastic or thermosett and are used in organic solvent born, waterborne, powder and radiation-curable coatings
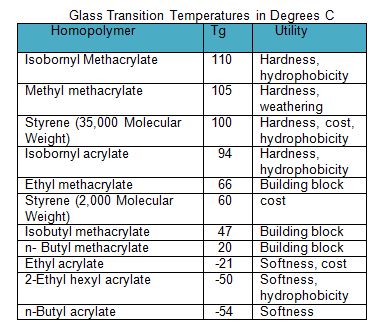
Three broad classes of liquid coatings utilizing acrylic resins include thermoplastic, thermoset and waterborne. Many acrylic resins may also include other vinyl monomers such as styrene or vinyl acetate primarily to reduce cost. Acrylic monomers have a lower Tg than their analogous methacrylate monomers (for example compare the Tg for n-butyl acrylate versus n-butyl methacrylate see Table I and Table II). As Table II suggests, the glass transition temperature of the monomers selected for synthesis of a resin can be selected to enhance multiple properties that may include weather resistance, moisture resistance, oxygen permeability, flexibility reactivity, cure and hardness. In addition, acrylics can be functionalized with a variety of monomers to provide improved adhesion to metal, or to react for example with aminoplast or isocyanate crosslinkers.

Thermoplastic acrylic polymers (TPA) in general have excellent properties including exterior durability. Such resins were widely used in automotive OEM and Refinish topcoats from the 50’s to the 70’s, but their use has dramatically declined due to the high molecular weight necessary to provide properties, they require a high amount of organic solvent to enable air atomized spray application. Accordingly these paints apply at about 20% weight solids. Thermoplastic resins typically use a high level of methyl methacrylate in their polymer backbone to provide excellent hardness and exterior durability.
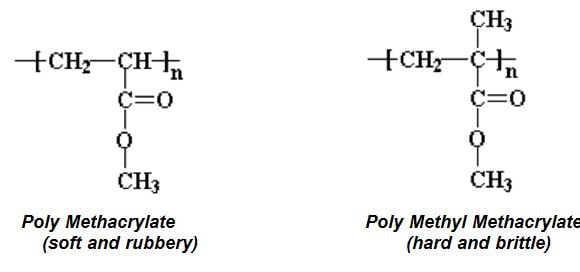
Thermosetting acrylic resins (TSA) are designed with functional monomers to either react with themselves when exposed to heat or moisture, or with that of a cross-linker to form a cross-linked film. Thermoset resins as a group are lower molecular weight and thus have higher application solids. Once cross-linked, as a class they offer films with excellent resistance to organic solvents, moisture and UV light and do not soften appreciably when exposed to moderately high temperatures as thermoplastics do. An example of acrylic monomers with functional groups that can be used to functionalize acrylic polymers to provide properties such as crosslinking, self-crosslinking, improved adhesion or pigment wetting are provided in Table III.
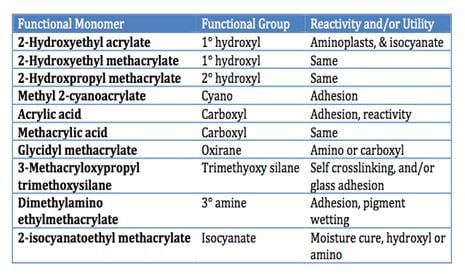
Being able to functionalize an acrylic resin with a wide range of reactive moieties provides the ability to tailor the performance of the resin backbone to provide improved adhesion over a variety of substrates, improved pigment wetting and/or the ability to provide crosslinking or self-crosslinking. Other acrylic monomers are also available to impart sulfonic acid, or phosphoric acid functionality to the acrylic resin.
Being able to functionalize an acrylic resin with a wide range of reactive moieties provides the ability to tailor the performance of the resin backbone to provide improved adhesion over a variety of substrates, improved pigment wetting and/or the ability to provide crosslinking or self-crosslinking. Other acrylic monomers are also available to impart sulfonic acid, or phosphoric acid functionality to the acrylic resin.
Carbamate functional acrylics can also be made for example by reacting an isocyanate functional acrylic with hydroxypropyl carbamate. Many of the acrylics in the category of functionalized acrylic resins are used in automotive OEM and refinish clearcoats to provide an excellent combination of mar resistance, chemical resistance and light stability.
To read the rest of Ron’s article, click here to head over to UL Prospector.