Coatings that offer a hydrophobic (EU) or superhydrophobic surface can impart multiple advantages to the coating surface and substrate they are applied to. Advantages may include decreased dirt retention, self-cleanability, improved moisture and corrosion resistance, as well as extended life expectancy of the coating and substrate. To fully explain and quantify hydrophobicity, it is necessary to define the relationship between contact angle and the hydrophobic/hydrophilic (EU) character of a surface.
Figure 1 – Contact Angle for Hydrophobic Coating Surface and a Hydrophilic Coating Surface
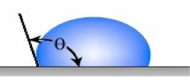
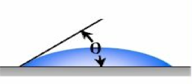
Figure 2 – Contact Angle and Superhydrophobicity
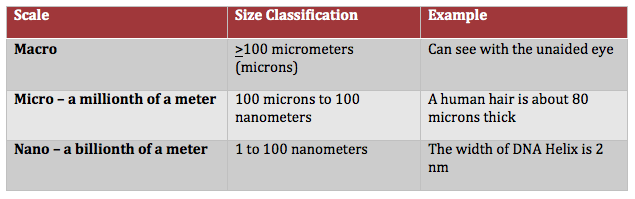
Accordingly, the surface characteristics of a coating can range from being hydrophilic (water-loving) to superhydrophobic (or highly water repellent). Several factors impact the contact angle of a water drop on the surface of a coating. These include the macro, micro, nano-surface profile, and the surface tension of the coating on which the water droplet is resting. Surface tension is the elastic tendency of liquids that make them acquire the least surface area possible.
To read the full article written by Ron Lewarchik, Chemical Dynamics President, on UL Prospector, click here.








 Since visible
Since visible 






